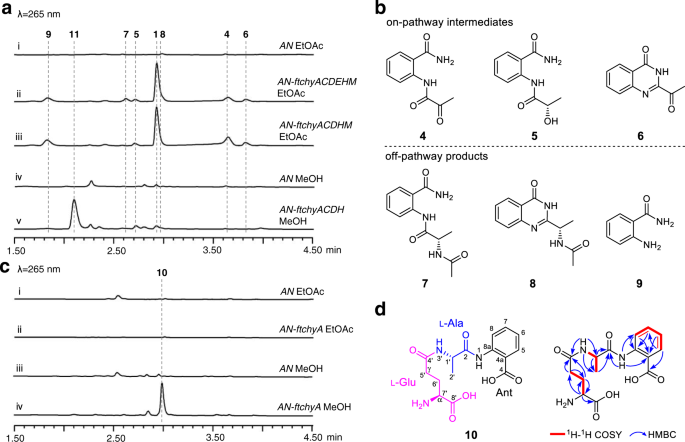
Genome mining reveals the ftchy cluster in F. tricinctum
To make clear the meeting equipment for the 4(3H)-quinazolinone scaffold synthesis of 1, we mined the chy homologue gene cluster from the Fusarium sp. genome database in our lab. A extremely homologous cluster (ftchy) was recognized from F. tricinctum CGMCC 3.4731 (Fig. 2a and Supplementary Desk 3). The ftchy cluster shares comparable gene composition and group with the chy cluster from P. chrysogenum and F. graminearum. To substantiate that the ftchy cluster is chargeable for the synthesis of 1, we constructed gene expression mixture plasmids for heterologous manufacturing in A. nidulans (Supplementary Fig. 4). As proven in Fig. 3a, i, ii, 1 was efficiently produced and purified from the AN-ftchyACDEHM transformant, and its construction was confirmed by NMR analyses (Supplementary Desk 5 and Supplementary Figs. 46, 47).
a LC-MS analyses of the A. nidulans transformant tradition extracts. b Chemical buildings of the compounds remoted from the AN-ftchyACDEHM transformant. c LC-MS analyses of the AN-ftchyA transformant tradition extracts. d Chemical construction of 10 displaying it’s a linear γ-l-glutamyl-l-alanyl-anthranilate tripeptide.
Along with 1, six extra compounds (4–9) had been produced by pressure AN-ftchyACDEHM (Fig. 3a, ii), albeit with decrease yields. We purified these compounds from the large-scale fermentation of AN-ftchyACDEHM and confirmed their buildings by NMR analyses (Supplementary Tables 8–13 and Supplementary Figs. 58–69), that are proven in Fig. 3b. Feeding the ftchyA KO pressure (AN-ftchyCDEHM) with these compounds demonstrated that 4, 5 and 6 might restore the manufacturing of 1, whereas 7, 8 and 9 had been all off-pathway merchandise (Supplementary Fig. 5). Though the beforehand recognized on-pathway intermediate 2 was not detected in pressure AN-ftchyACDEHM, the manufacturing of 7 strongly indicated that 2 ought to be transformed to 7 by an unknown acetyltransferase of A. nidulans20.
Structural evaluation indicated that 1 and 5 are the lowered merchandise of 6 and 4, respectively, the response of which is probably catalysed by the quick chain dehydrogenase/reductase (SDR) encoded by the gene ftchyC. Certainly, when purified ftChyC (~31 kDa, from Escherichia coli, Supplementary Fig. 6) was incubated with 6 and 4 within the presence of the cofactor NADPH, 1 and 5 had been detected (Supplementary Fig. 7). Nevertheless, reverse dehydrogenation by ftChyC in direction of 1 and 5 was not noticed (Supplementary Fig. 7). These outcomes present that ftChyC is a promiscuous reductase that may cut back 6 and 4, and the discount of 6 to 1 is probably the ultimate step within the synthesis of 1.
In vivo heterologous expression of ftchyA in A. nidulans unexpectedly produces linear tripeptide 10
To dissect the artificial steps of 1, we first centered on the two-module NRPS ftChyA. The gene ftchyA was cloned from the gDNA of F. tricinctum and heterologously expressed in A. nidulans beneath the management of the gpdA promoter (Supplementary Fig. 4). After 3.5 days of stable medium tradition adopted by extraction with ethyl acetate (EtOAc), nonetheless, no anticipated product, akin to 3, was detected (Fig. 3c, i, ii). Alternatively, we used methanol (MeOH) because the solvent to extract the tradition, and one compound (10) with m/z 338 [M + H]+ was produced by AN-ftchyA (Fig. 3c, iii, iv). Purification of 10 from the large-scale fermentation of AN-ftchyA and subsequent structural dedication by NMR analyses (Supplementary Desk 14 and Supplementary Figs. 70–75) confirmed that 10 was a linear tripeptide consisting of l-Glu, l-Ala and Ant (Fig. 3d).
It’s value mentioning that, in accordance with the important thing heteronuclear a number of bond correlation (HMBC) of NH-3′ with C-1′, C-4′ and C-2′ (Fig. 3d), 10 encompasses a uncommon amide bond constituted by the γ-COOH of l-Glu and the α-NH2 of l-Ala. Such a amide bond is seldom present in nonribosomal peptides, and sporadic examples akin to butirosin and microcystin LR from micro organism and δ-l-α-aminoadipyl-l-cysteinyl-d-valine (ACV tripeptide, a penicillin precursor) from fungi, have been reported21,22,23. The manufacturing of 10 by AN-ftchyA exhibits an uncommon instance by which fungal two-module NRPS synthesizes a tripeptide in vivo24, which is considerably totally different from the classical fungal two-module NRPSs that often generate diketopiperazine backbones25,26,27,28,29.
ftChyA biochemical assays unveil an uncommon two-module NRPS meeting course of for the synthesis of 10 and three
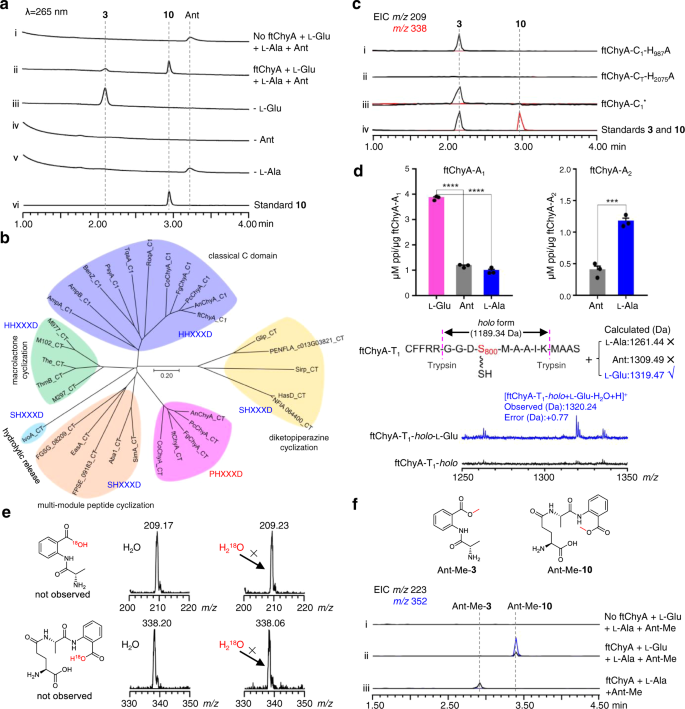
To make clear the weird relationship between ftChyA and 10, we subsequent tried to analyze the operate of ftChyA in vitro. Intron-free ftchyA was cloned, and ftChyA was expressed and purified from E. coli (~265 kDa, Supplementary Figs. 6, 8). Apo-ftChyA was enzymatically transformed into its holo kind through the use of phosphopantetheinyl transferases NpgA and CoA30. When 5 μM holo-ftChyA was incubated with 1 mM of the substrates l-Glu, l-Ala and Ant, in addition to the cofactors ATP and Mg2+, two merchandise had been produced. The key one is in line with normal 10; one other minor one with m/z 209 [M + H]+ is in line with anticipated 3 (Fig. 4a, i, ii, vi). Furthermore, 10 was steady beneath the response situations (Supplementary Fig. 9) indicating that the minor compound isn’t from 10; it is usually the product of ftChyA. Extra mixtures confirmed that (1) the elimination of l-Glu solely abolished the manufacturing of 10 (Fig. 4a, iii); and (2) the elimination of l-Ala or Ant abolished each 10 and the minor compound (Fig. 4a, iv, v). These outcomes clearly demonstrated that l-Ala and Ant are the constructing blocks of the minor compound. The minor compound was lastly purified from large-scale biochemical assays, and its construction was confirmed to be l-alanyl-anthranilate dipeptide 3 by NMR analyses (Supplementary Desk 7 and Supplementary Figs. 53–57).
a Biochemical affirmation of ftChyA synthesizing 10 and 3. b Phylogenetic evaluation of fungal NRPS C domains displaying that ftChyA-CT is separated into an impartial clade. The C area protein sequences used for phylogenetic evaluation are listed in Supply Information file. c ftChyA-C1 and ftChyA-CT area mutations verify that the C1 area is chargeable for amide bond formation between l-Glu and l-Ala of 10 and that CT is chargeable for amide bond formation between l-Ala and Ant to launch 10 and 3. d ATP-PPi launch assay and MALDI-TOF MS evaluation of the A domains for substrate recognition. Information are proven because the imply ± SEM of three impartial experiments. ***P < 0.001, ****P < 0.0001 (ftChyA-A1, P = 1.5e-6 between l-Glu and Ant, P = 4.5e-6 between l-Glu and l-Ala; ftChyA-A2, P = 0.0008 between l-Ala and Ant), unpaired two-tailed Pupil’s t take a look at. e Biochemical assays of ftChyA in H218O-Tris buffer confirming that water isn’t concerned within the formation of 10 and 3. f In vitro assays of ftChyA with l-Glu, l-Ala and Ant-Me. The extracted ion chromatograms (EICs) had been extracted at m/z 223 [M + H]+ for Ant-Me-3 and m/z 352 [M + H]+ for Ant-Me-10.
The above outcomes (1) set up the connection of ftChyA with 10 and signify the biochemical proof that fungal two-module NRPS synthesizes a tripeptide; and (2) show that ftChyA has a further capability to kind dipeptide 3; nonetheless, beneath the equal provide of all substrates, ftChyA favours the synthesis of tripeptide 10. This may be the rationale why 3 was not detected in AN-ftchyA in vivo. Subsequently, we moreover fed Ant (200 μM to 1 mM) into AN-ftchyA, and 3 was detected by LC-MS evaluation (Supplementary Fig. 10).
ftChyA-CT catalyses the discharge of the net γ-l-glutamyl-l-alanyl-S-T2 or l-alanyl-S-T2 by way of the offline anthranilate
Simultaneous manufacturing of 10 and 3 by ftChyA implies its distinctive catalytic mechanism, particularly amide bond formation in 10 and 3, for linear tripeptide and dipeptide synthesis. Phylogenetic evaluation (Fig. 4b) and sequence similarity community (SSN) evaluation (Supplementary Fig. 11) of ftChyA-C1 and ftChyA-CT with different recognized fungal NRPS C domains11,25,31,32,33,34,35,36 confirmed that (1) ftChyA-C1 comprises the usual H986H987xxxD991 motif (Supplementary Fig. 12), which belongs to the canonical extension clade of amide bond formation; and (2) ftChyA-CT and its homologous enzymes from different chy clusters (Supplementary Fig. 13) function the P2074H2075xxxD2079 motif (the place the primary residue His was changed by Professional; Supplementary Fig. 14) and are clustered into an impartial clade with an unknown operate.
To make clear the roles of those two C domains, we carried out site-directed mutagenesis on the lively residues H987 of ftChyA-C1 and H2075 of ftChyA-CT (Supplementary Figs. 6, 8). The in vitro assays confirmed that (1) the mutation H987A of ftChyA-C1 abolished the formation of 10; nonetheless, it didn’t have an effect on the manufacturing of 3 (Fig. 4c, i); and (2) ftChyA-CT H2075A abolished the manufacturing of 10 and 3 (Fig. 4c, ii). To exclude the attainable complementary results of H986 on the H987A mutation in ftChyA-C1, we additional constructed the A986A987xxxA991 (ftChyA-C1*) mutant; nonetheless, ftChyA-C1* retained the flexibility to generate 3 (Fig. 4c, iii). These outcomes (1) show that ftChyA-C1 doesn’t take part within the synthesis of 3, nonetheless, it’s chargeable for the era of 10. Thus, ftChyA-C1 is chargeable for amide bond formation between l-Glu and l-Ala of 10; (2) strongly point out that ftChyA-CT not solely catalyses amide bond formation between l-Ala and Ant of 10 and 3 but additionally catalyses the discharge of 10 and 3.
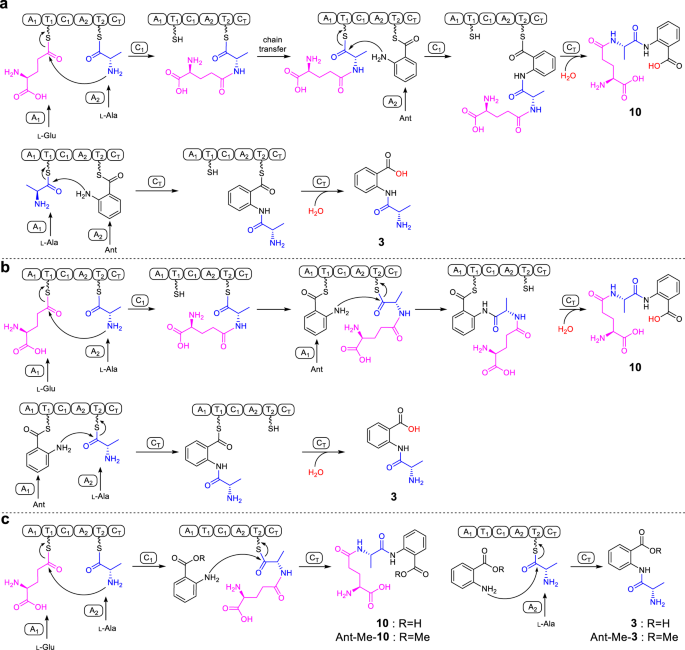
Primarily based on the canonical NRPS meeting rule and the order of amino acids in 10, ftChyA-A1 ought to acknowledge l-Glu for the synthesis of 10 (Fig. 5a). Nevertheless, for the synthesis of 3, ftChyA-A1 wants to acknowledge l-Ala (Fig. 5a). Subsequently, the operate of ftChyA-A1 for 10 and 3 appears inconsistent. Furthermore, the chain switch from γ-l-glutamyl-l-alanyl-S-T2 to γ-l-glutamyl-l-alanyl-S-T1, in addition to the repeat recognition of Ant by ftChyA-A2, are each required to assist the synthesis of 10 (Fig. 5a). Subsequently, the proposed mechanism a for 10 and 3 is questionable. Alternatively, in accordance with the lately reported uncommon pass-back mechanism of NRPS from α-proteobacteria37, mechanism b (no chain switch concerned) was additionally proposed (Fig. 5b). On this mannequin, ftChyA-A2 uniformly acknowledges l-Ala for 10 and 3; nonetheless, ftChyA-A1 wants to acknowledge l-Glu and Ant for 10 and Ant for 3. It’s value mentioning that though mechanisms a and b are totally different, the releases of the proposed γ-l-glutamyl-l-alanyl-anthranilyl-S-T and l-alanyl-anthranilyl-S-T to kinds 10 and 3 are all mediated by water.
To check these two hypotheses, we first expressed and purified stand-alone ftChyA-A1 (~86 kDa, Supplementary Fig. 6) from E. coli, which was then subjected to an ATP-inorganic pyrophosphate(PPi) launch assay38. In distinction to l-Ala and Ant, ftChyA-A1 favoured l-Glu, the place the specificity distinction was important (Fig. 4d). Extra matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) evaluation confirmed that, beneath the equal aggressive experiment, ftChyA-A1 solely acknowledged and loaded l-Glu to the Ser binding web site (S800) of ftChyA-T1–holo (Fig. 4d). The following ATP-PPi launch assay of ftChyA-A2 (~108 kDa, with a maltose binding protein (MBP)-tag, Supplementary Fig. 6) in direction of l-Ala and Ant confirmed that l-Ala was the substrate of ftChyA-A2 (Fig. 4d). These outcomes show that each mechanisms a and b are usually not affordable for 10 and 3, thus excluding that the formation of 10 and 3 was mediated by water. Certainly, when the biochemical assays of ftChyA had been carried out in H218O-Tris-HCl buffer, the incorporation of 18O into 10 and 3 was not noticed, and the molecular weights of 10 and 3 weren’t elevated (Fig. 4e).
Contemplating the outcomes of C area and A site, a mannequin for the synthesis of 10 and 3 by ftChyA was proposed (Fig. 5c). On this mannequin, Ant doesn’t have to be loaded by ftChyA-A1 or ftChyA-A2 to the T domains; the net γ-l-glutamyl-l-alanyl-S-T2 or l-alanyl-S-T2 is attacked by the offline Ant to launch 10 or 3, respectively, the place the method is catalysed by ftChyA-CT. To check this speculation, blocking the carboxyl group of Ant is required, which guidelines out the opportunity of A domains loading the substrate. The best compound is anthranilate methyl ester (Ant-Me). When Ant was changed by Ant-Me in ftChyA assays, the corresponding merchandise Ant-Me-10 and Ant-Me-3 had been detected by LC-MS and HRMS analyses (Fig. 4f, Fig. 5c and Supplementary Figs. 44, 45), respectively. We additional constructed, expressed, and purified CT domain-truncated ftChyAΔCT (A1-T1-C1-A2-T2, ~218 kDa, Supplementary Figs. 6, 8) and stand-alone ftChyA-CT (~55 kDa, Supplementary Fig. 6) from E. coli. When ftChyAΔCT was incubated with ftChyA-CT, the formation of 10 and 3 was noticed (Supplementary Fig. 15). The yields of 10 and 3 elevated when the focus of ftChyA-CT elevated (Supplementary Fig. 15). These outcomes show that ftChyA-CT is a singular C area, representing an uncommon operate of fungal two-module NRPS C domains34. The assault by the offline free amino acid on the net T domain-tethered dipeptide or T domain-tethered amino acid to launch the formation of the linear tripeptide or dipeptide is catalysed.
ftChyD makes use of inorganic ammonium ions or the amide of l-Gln to catalyse the amidation of 10 to 11 and three to 2
Affirmation of ftChyA as an uncommon tripeptide synthase inspired us to analyze the subsequent steps of 1. The following focus is the amidation of the Ant fragments of 10 and 3, the place the candidate enzyme for this modification is ftChyD. ftChyD is an asparagine synthase protein belonging to the category II glutamine amidotransferase household;39 it comprises an intact N-terminal catalytic cysteine (Cys2) residue and C-terminal synthase area to bind ATP and substrate (Supplementary Fig. 16)40.
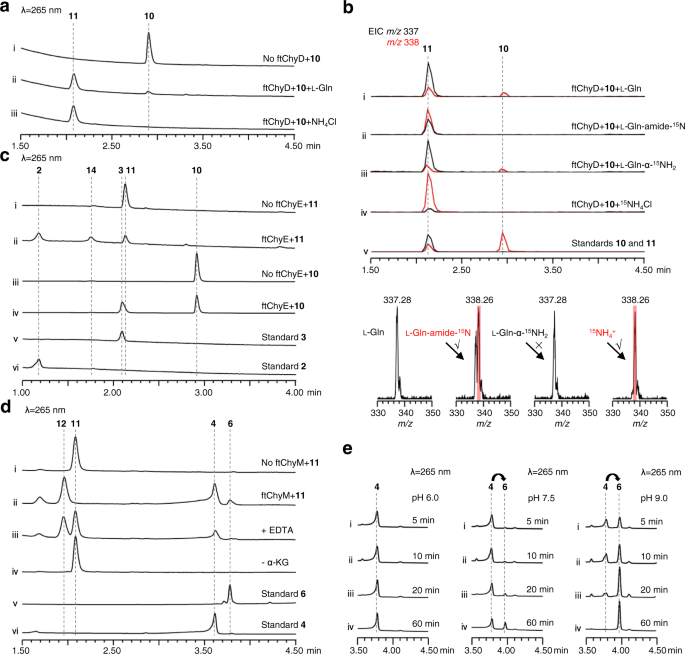
To research the flexibility of ftChyD (~78 kDa) to catalyse the amidation of 10 and 3, it was expressed and purified from E. coli (Supplementary Fig. 6). With l-Gln and ATP, ftChyD transformed 10 and 3 into the corresponding amidation merchandise 11 (m/z 337 [M + H]+) and 2 (m/z 208 [M + H]+), respectively (Fig. 6a, i, ii, and Supplementary Fig. 17a). These two merchandise had been purified from the large-scale in vitro assays, and their buildings had been confirmed by NMR analyses (Supplementary Tables 6, 15 and Supplementary Figs. 48–52 and Figs. 76–81). Additional 15N-labelled l-Gln assays confirmed that the amide of l-Gln was the amide donor (Fig. 6b, i, ii, and Supplementary Fig. 17b–d), whereas the α-NH2 of l-Gln couldn’t be included into 11 and 2 (Fig. 6b, iii, and Supplementary Fig. 17b–d). Apparently, once we used NH4Cl to switch l-Gln, the formation of 11 and 2 was enhanced (Fig. 6a, iii, and Supplementary Fig. 17a), supporting that ftChyD favours inorganic ammonium ions because the amide donor. The incorporation of NH4+ into 11 and 2 was additionally demonstrated by 15NH4Cl (Fig. 6b, iv, and Supplementary Fig. 17b–d). The kcat/OkM calculation confirmed that the catalytic effectivity of ftChyD in direction of 10 was almost 36-fold higher than that in direction of 3 (Supplementary Fig. 18), which signifies that 10 is the optimum substrate of ftChyD. These outcomes show that the N-3 of the 4(3H)-quinazolinone scaffold in 1 comes from the inorganic ammonium ions or the amide-N of l-Gln, which is totally different from the mechanisms proven in Fig. 1c, d.
a ftChyD makes use of NH4Cl or l-Gln to catalyse the amidation of 10 to kind 11. b LC-MS evaluation of the incorporation of 15N into 11. The EICs had been extracted at m/z 338 [M + H]+ for 10 and 15N-labelled 11, and m/z 337 [M + H]+ for 11. c ftChyE catalyses the hydrolysis of 11 and 10 to kind 2 and 3, respectively. d ftChyM is an α-KGD that catalyses the C-N bond oxidative cleavage of 11 to kind 4 by way of a attainable intermediate 12. e Conversion of 4 to 6 exhibits the alkaline-induced spontaneous C-2-N-3 bond closure.
ftChyE is a tripeptide hydrolase that converts 11 to 2
A earlier feeding experiment confirmed that 2 is the precursor of 118; thus, elimination of the l-Glu fragment of 11 by hydrolysis to present 2 ought to be investigated. We centered on ftChyE, though it was beforehand annotated as a malonyl transferase18. Nevertheless, conserved area evaluation confirmed that it’s the glutaminase subunit of a category I glutamine amidotransferase (GATase)39. Sequence alignment of ftChyE with its homologous enzymes and different recognized class I GATases confirmed that ftChyE comprises the intact catalytic triad (Cys102-His189-Glu191, C-H-E, Supplementary Fig. 19) for glutaminase exercise, which hydrolyses glutamine to glutamate and nascent ammonia41.
To check whether or not ftChyE might hydrolyse 11 to 2, ftChyE (~74 kDa, with an MBP tag, Supplementary Fig. 6) was expressed and purified from E. coli. When 11 was incubated with ftChyE, the manufacturing of 2 (and its spontaneous cyclization product 14, see beneath) was detected (Fig. 6c, i, ii). The substrate tolerance of ftChyE hydrolysing 10 to present 3 was additionally investigated (Fig. 6c, iii, iv). Affirmation of the operate of ftChyE (1) establishes the bridge between the tripeptide and dipeptide within the synthesis of 1 and (2) represents the distinctive operate of sophistication I GATase as tripeptide hydrolase.
ftChyM is an uncommon α-KGD that catalyses the C-N bond oxidative cleavage of 11 to kind 4
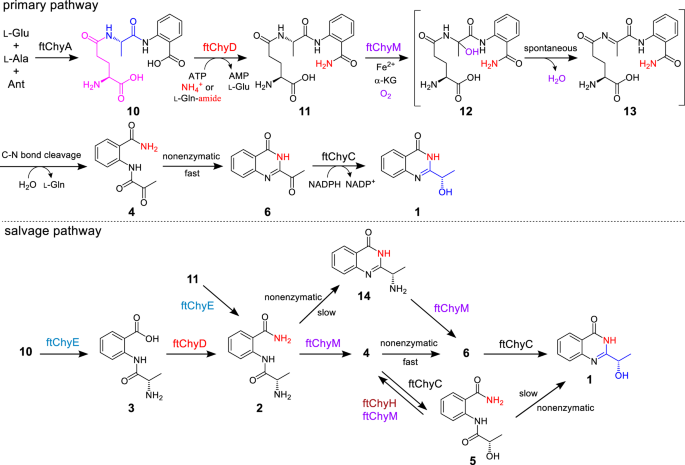
Though ftChyE-catalysed hydrolysis of 11 to 2 was confirmed, earlier transcription evaluation of the chy cluster in F. graminearum confirmed that chyE was expressed at a decrease degree than the remaining cluster genes19, suggesting that chyE isn’t the important gene for the synthesis of 1. To check this speculation, we constructed the AN-ftchyACDHM transformant and located that elimination of ftchyE doesn’t abolish the manufacturing of 1 (Fig. 3a, iii), which means that the ftChyE-participating pathway (by way of 2, together with 11 → 2 and 10 → 3 → 2) is probably not the first route however the salvage route for the synthesis of 1 (Fig. 7).
Since 4 is the on-pathway intermediate of 1 (Fig. 3b and Supplementary Fig. 5), a C-N bond cleavage course of from 11 to 4 ought to happen (Fig. 7). By this pathway, the oxygen atom of the α-OH group (C-1′-OH) of 1 may very well be from water (Fig. 7 and Supplementary Fig. 25a). To check this speculation, we incubated AN-ftchyACDEHM in H218O-medium, and the incorporation of 18O into 1 was noticed by LC-MS (Supplementary Fig. 20). The remaining unidentified genes within the ftchy cluster had been ftchyM and ftchyH. Conserved area evaluation confirmed that ftChyH is a flavin-dependent oxidase that comprises a berberine bridge enzyme (BBE) conserved area (Supplementary Fig. 21a) and belongs to the BBE-like oxidase superfamily42. BBE-like oxidases often catalyse dehydrogenation-mediated C-C or C-N bond formation reactions throughout pure product biosynthesis42. Subsequently, ftChyH was proposed to be chargeable for 4(3H)-quinazolinone ring formation by way of C-2-N-3 bond closure. Alternatively, ftChyM confirmed 31%/45% id/similarity to GA4 desaturase, an α-KGD that converts GA4 to GA7 by forming a C-1-C-2 double bond throughout gibberellic acid biosynthesis43. Conserved area evaluation confirmed that the HxD motif (which binds Fe2+) and the RxS motif (which binds α-ketoglutarate, α-KG) had been intact in ftChyM (Supplementary Fig. 21b). Additional sequence clustering evaluation confirmed that ftChyM and its homologous household proteins signify a singular α-KGD cluster in fungi (Supplementary Fig. 22). Subsequently, we reasoned that ftChyM may catalyse the C-N bond oxidative cleavage of 11 to kind 4.
To check this speculation, ftChyM (~46 kDa, Supplementary Fig. 6) was expressed in E. coli and purified through the use of dithiothreitol (DTT; 1 mM ultimate focus)-containing Tris-HCl buffer, which ensured that the FeII core was not inactivated as a consequence of oxidation. When ftChyM was incubated with 11 within the presence of α-KG, Fe2+ and vitamin C, 4 and 6¸had been produced (Fig. 6d, i, ii). The addition of EDTA (5 mM) to bind Fe2+ tremendously decreased the exercise of ftChyM (Fig. 6d, iii). Furthermore, the elimination of α-KG and formation of 4 and 6 weren’t noticed (Fig. 6d, iv), thus suggesting that ftChyM, as a normal α-KGD, requires these cofactors for enzymatic exercise. We additionally investigated the ftChyM-catalysed deamination of 2, and the time-course evaluation confirmed that the chemical conversion of ftChyM in direction of 2 is slower than that in direction of 11 (Supplementary Fig. 23a, c). Apparently, when 5 was incubated with ftChyM, albeit with low yields, the formation of 4 and 6 was additionally noticed (Supplementary Fig. 23b), which confirmed that ftChyM has a further capability to catalyse dehydrogenation. These outcomes show that ftChyM-catalysed conversion of 11 to 4 is the indispensable route for 1 synthesis. Certainly, additional elimination of the ftchyM transformant AN-ftchyACDH led to the buildup of 11 however abolished the manufacturing of 1 (Fig. 3a, iv, v).
Aside from 4 and 6, compound 12 with m/z 353 [M + H]+ (11 plus 16 Da) was noticed within the ftChyM-catalysed assay (Fig. 6d). We initially tried to purify 12; nonetheless, it spontaneously and fully transformed to 4 and 6 inside 8 h (Supplementary Fig. 23c, d). When the ftChyM-catalysed assay was carried out in ~80% H218O-Tris HCl buffer, almost ~73% of 18O-labelled 4 and 6 (in sum) was detected (Supplementary Fig. 24); nonetheless, the incorporation of 18O into 12 was not noticed (Supplementary Fig. 24). Subsequently, 12 ought to be the hydroxylation intermediate of 11 catalysed by ftChyM, which is probably fashioned by the next steps (Fig. 7 and Supplementary Fig. 25a, b): (1) the reactive [FeIV = O] species of ftChyM homolytically breaks the inactive Cα-Hα bond of the l-Ala moiety in 11 to supply the 11 Cα radical and (2) the lowered FeIII-OH offers a hydroxyl radical to yield hemiaminal intermediate 1244,45. As soon as 12 is produced, its dehydration to kind iminium intermediate 13, in addition to the next hydrolysis of 13 to present 4, are possible spontaneous steps (Fig. 7 and Supplementary Fig. 25a, b)44,46.
The α-carbonyl group in 4 tremendously promotes spontaneous cyclization to kind the 4(3H)-quinazolinone scaffold
Though 4 and 6 had been concurrently produced within the ftChyM-catalysed assays, we reasoned that 6 is the spontaneous cyclization product of 4. That is probably because of the electron-withdrawing impact of the α-carbonyl group of 4, which makes the C-2 centre extra electron poor, thereby inducing C-2-N-3 closure to kind the 4(3H)-quinazolinone scaffold (Supplementary Fig. 25c). To check this speculation, 4 was incubated alone in Tris-HCl buffer at a pH of seven.5, and the time-dependent formation of 6 was clearly noticed (Supplementary Fig. 26a). The addition of ftChyM didn’t speed up this conversion (Supplementary Fig. 26b), which confirms that ftChyM doesn’t take part within the subsequent cyclization step (Fig. 7). Notably, the conversion charge of 4 to 6 happens with a rise within the pH worth of the buffer (Fig. 6e), which signifies that the hydroxyl anion from the alkaline buffer may be useful for the proposed C-2-OH departure (Supplementary Fig. 25c).
We additionally investigated the spontaneous transformations of 2 and 5 into their corresponding cyclization merchandise 14 and 1, respectively (Fig. 7, Supplementary Desk 16 and Supplementary Figs. 82, 83). Though these cyclization merchandise had been noticed, the conversions had been sluggish (Supplementary Fig. 27), which exhibits that the α-carbonyl group of 4 tremendously promotes spontaneous cyclization for the synthesis of 4(3H)-quinazolinone scaffold 6.
ftChyH ensures the availability of 4 by correcting the extra discount response carried out by ftChyC
The above outcomes confirmed that the formation of the 4(3H)-quinazolinone scaffold in 1 is an α-carbonyl group formation-driven nonenzymatic cyclization course of. This statement excludes the BBE oxidase ftChyH, the best candidate that was beforehand proposed to be chargeable for this key step. Thus, the precise operate of ftChyH ought to be reconsidered. We constructed AN-ftchyACDM and located that 5 gathered on this transformant (Supplementary Fig. 28a), which extremely means that ftChyH catalyses the dehydrogenation of 5 to present 4.
To check this speculation, we tried to substantiate the operate of ftChyH utilizing a purified enzyme; nonetheless, this protein was not soluble in E. coli even when glutathione S-transferase (GST)-tagged or MBP-tagged ftChyH was constructed (Supplementary Fig. 29). Alternatively, yeast was used because the heterologous expression host, and the ftChyH cell-free extracts might catalyse the dehydrogenation of 5 to kind 4 and 6 (Supplementary Fig. 28b). When 5 was changed by 11 or 2, the era of 4 and 6 was not noticed (Supplementary Fig. 28b). These outcomes verify that ftChyH solely catalyses the dehydrogenation response and may appropriate the extra discount of ftChyC in direction of 4, guaranteeing the first pathway (4 → 6) within the fast development of the 4(3H)-quinazolinone scaffold. Different branched pathways, relying on the nonenzymatic cyclization (5 → 1, Supplementary Fig. 27) or promiscuous substrate selectivity of ftChyM (14 → 6 → 1, Supplementary Fig. 30), had been additionally confirmed throughout the synthesis of 1 (Fig. 7).
As proven in Fig. 7, synthesis of 1 from 10 represents another route for producing the 4(3H)-quinazolinone scaffold. Aside from the inorganic substrates are concerned in 1 synthesis, the principle sudden meeting equipment is that l-Glu is first recruited by ftChyA to synthesize 10, nonetheless, it’s then eliminated as l-Gln by ftChyM throughout the publish tailoring steps of 1. This appears a redundant course of for the formation of 1; nonetheless, it’s notably value mentioning that the generated l-Gln from ftChyM response may very well be recaptured and hydrolysed by ftChyD to yield l-Glu and ammonium ions, the place these two merchandise might re-participate in ftChyA-catalysed 10 formation and ftChyD-catalysed amidation reactions, respectively. Subsequently, from this attitude, an environment friendly self-circulation system amongst ftChyA, ftChyD and ftChyM-catalysed reactions has been established throughout the artificial technique of 1 (Supplementary Fig. 84).
On this work, by way of in vitro investigation of the pathway of 1, sudden meeting equipment for the synthesis of the 4(3H)-quinazolinone scaffold was unveiled and biochemically confirmed, which importantly (1) reveals a fungal two-module NRPS with an uncommon CT area catalysing tripeptide formation; (2) reveals that the nitrogen supply for N-3 is inorganic ammonium ions or amide of l-Gln; and (3) demonstrates an uncommon α-KGD catalysing the C-N bond oxidative cleavage of a tripeptide to kind a dipeptide. Our examine demonstrates a singular launch and tailoring mechanism of nonribosomal peptides and represents another native artificial logic within the development of 4(3H)-quinazolinone scaffolds.