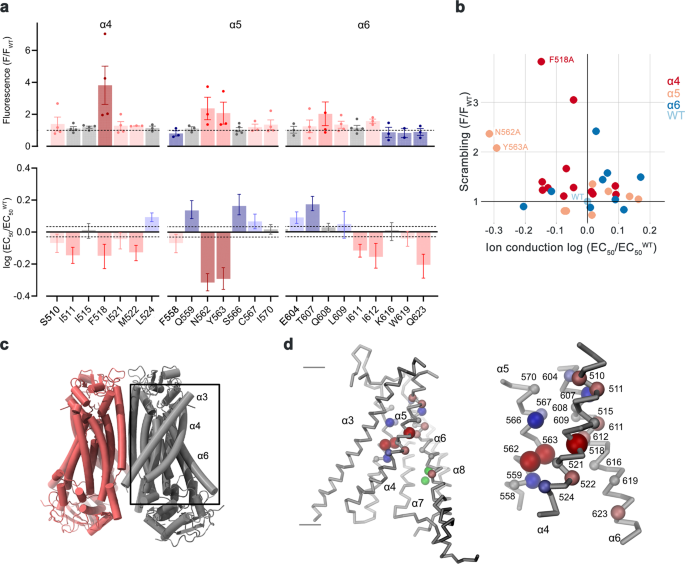
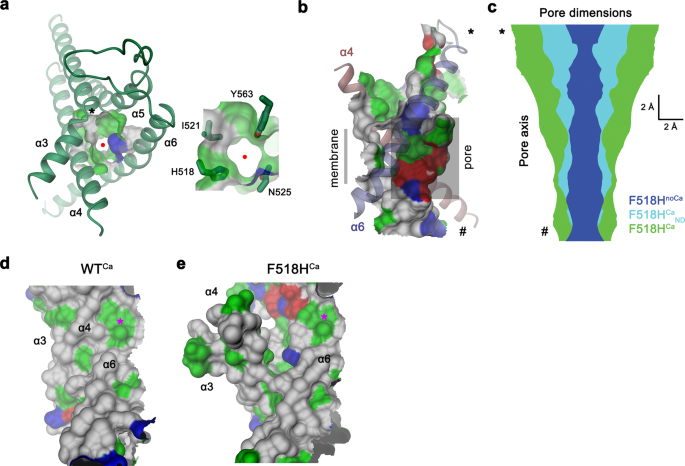
Scanning mutagenesis of the pore area
On the onset of our research, we have been within the relevance of residues situated on α-helices 4–6, that are buried within the widespread core of the presumably closed permeation path in WT buildings, for conformational transitions that facilitate lipid scrambling and ion conduction. To this finish, we characterised alanine mutants with regard to each practical properties (Fig. 1, Supplementary Fig. 1). We investigated the impact of mutations on lipid conduction utilizing a mobile scrambling assay. Protein constructs have been expressed in a cell line carrying a genetic knock-out of TMEM16F (generously offered by Dr. Huanghe Yang), which itself lacks any Ca2+-dependent scrambling exercise41. In these experiments, we quantified the steady-state fluorescence ensuing from the binding of labeled annexin V to uncovered phosphatidylserine at resting Ca2+-concentrations and the fluorescence enhance 780 s after the addition of ionomycin, which elevates the intracellular Ca2+ degree (Fig. 1a, Supplementary Fig. 1a). This assay, which is especially fitted to the detection of mutants with strongly activating phenotype that present elevated fluorescence in comparison with WT already at resting Ca2+ concentrations, revealed elevated actions for Phe 518 on α4, Asn 562 and Tyr 563 on α5, and Gln 608 and Ile 612 on α6 (Fig. 1a, b, Supplementary Fig. 1a). In complementary research based mostly on electrophysiology, we anticipated mutations that stabilize an activated state to extend, and conversely, ones that stabilize the resting state to decrease Ca2+-potency. Such habits was noticed in a earlier investigation of the chloride channel TMEM16A31. Our research revealed a predominant left-shift of the EC50 for positions on α4 and a right-shift for a number of mutants on α6, which are situated extracellular to the Ca2+-binding web site (Fig. 1a–d, Supplementary Fig. 1b). Notable exceptions on α6 concern Ile 611, Ile 612 and Gln 623, that are positioned on either side in relation to Gly 615, the pivot for the rearrangement of α6 in response to Ca2+-binding19 (Fig. 1a, Supplementary Fig. 1b). The magnitude of the shifts varies in dimension, deviating as much as 35% from the unique values when plotted on a logarithmic scale (Fig. 1a). Variations in each instructions are discovered for mutations on α5, which constitutes the middle of the subunit cavity18. On this helix, level mutations of residues Asn 562 and Tyr 563 lead to a two-fold lower of the EC50, revealing two positions the place sidechain truncations strongly destabilize the closed state (Fig. 1a, b, d).
a Alanine scan of residues lining the pore of the closed ion and lipid permeation area of TMEM16F. Prime, preliminary fluorescence in a cell-based lipid scrambling assay monitoring the binding of fluorescently tagged annexin V to phosphatidylserine on the cell floor. Values reflecting the lipid transport actions of TMEM16F mutants at resting Ca2+-concentrations have been normalized to the fluorescence of WT (dashed line). Information present imply of the displayed variety of experiments, errors are s.e.m. Backside, EC50 of Ca2+ activation recorded in excised patches. Information present EC50 values derived from a Hill-fit of dose-response curves proven in Supplementary Fig. 1b. Mutant EC50s are expressed as log-ratio in comparison with WT, errors are 95% confidence intervals. Dashed traces confer with the 95% confidence interval of WT. Colours mirror path and magnitude of change. b Scatter plot illustrating the connection between imply values for scrambling normalized to WT and log-fold adjustments within the EC50 for every mutant as depicted in a. Colours confer with the situation of the positioning of mutation. Information from chosen residues are labeled. c Common structure of TMEM16F (PDBID 6QPB). The field highlights the subunit cavity. d Cα illustration of the subunit cavity constituting the ion and lipid permeation area of TMEM16F (PDBID 6QP6) with Cα positions of mutated residues proven as spheres and coloured in line with the magnitude of the impact proven in a. Membrane boundaries are indicated. Inset (proper) exhibits blowup of the tightly packed area with residue variety of mutated websites indicated. Supply information are offered as a Supply Information file.
Together, each assays revealed an identical image of the energetic contributions of residues to the activation of TMEM16F. Most mutations affected each of its capabilities as ion channel and lipid scramblase in an identical method, with mutation of a gaggle of residues situated near the middle of the membrane to alanine exerting a robust stabilization of the open state (Fig. 1b–d). A number of of those residues conform with positions recognized in a current research based mostly on molecular dynamics simulations and mobile lipid transport assays that have been assigned a operate as intracellular gate40.
Purposeful properties of activating mutants
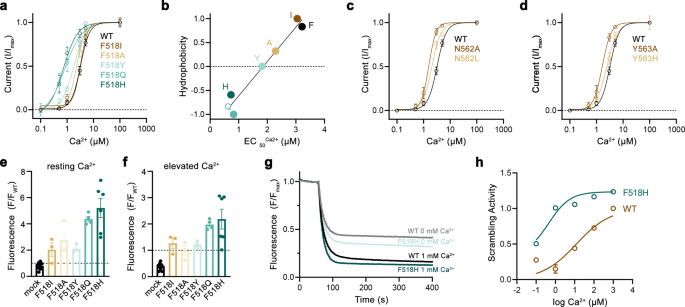
After figuring out positions the place sidechain truncations improve activation, we turned all for how the mutants act to stabilize the open state of TMEM16F. To this finish, we’ve got chosen the residues Phe 518 on α4 and Asn 562 and Tyr 563 on α5 for extra detailed investigations. For all three positions, we’ve got systematically studied level mutants with distinct sidechain properties to correlate the impact of the mutation on protein operate. Initially, we characterised ion conduction properties by patch-clamp electrophysiology after changing every of the three positions with amino acids of various hydrophilic character (Fig. 2a–d). For the α4 residue Phe 518, we’ve got investigated six mutants starting from the aliphatic isoleucine to the polar histidine (Fig. 2a, b). Amongst mutants of this place, we discover a linear correlation of the EC50 with the hydrophilicity of the sidechain alternative with histidine exerting the strongest impact, leading to a three-fold lower of the EC50 (i.e., from 3 to 1 µM, Fig. 2b). Remarkably, this correlation was solely noticed for Phe 518 and never for the 2 α5 residues Asn 562 and Tyr 563, the place the truncation to alanine had the biggest influence on calcium efficiency (Fig. 2c, d). An identical phenotype of Phe 518 mutants was discovered within the mobile scrambling assay, the place the alternative with polar sidechains elevated scrambling exercise at each, resting and elevated Ca2+ concentrations (Fig. 2e, f). Lastly, we investigated the Ca2+ dependence of lipid scrambling of reconstituted mutants of Phe 518 in vitro with an assay that was beforehand used to find out Ca2+-concentration relationships for lipid conduction of WT TMEM16F19 (Supplementary Fig. 2). In these experiments, we discovered a concordant Ca2+-dependence of scrambling as noticed for ion conduction with the mutant F518H exhibiting the strongest activating properties among the many investigated constructs (Fig. 2g, h, Supplementary Fig. 2). Collectively, our experiments have demonstrated that the alternative of Phe 518 by hydrophilic residues exerts a strongly stabilizing impact on the lively state of TMEM16F, which extends to each ion- and lipid conduction. Because the residue is situated on the interface between α-helices 4 and 6, these outcomes additionally emphasize the position of a practical crosstalk between each helices throughout Ca2+-activation. F518, Y563 and N562 cluster throughout the narrowest a part of the presumed area of ion and lipid permeation. Consequently, the practical phenotype of their mutation hints in direction of a conformational change on this area that takes place throughout activation and that was not noticed within the Ca2+-bound buildings of WT19,37.
a Ca2+-concentration-response relationships of mutants of Phe 518 measured in inside-out patches. b EC50 values of Ca2+ plotted in opposition to the normalized hydrophobicity (Eisenberg and Weiss Scale) of the respective Phe 518 mutations. Line exhibits a match to the information. c Ca2+-concentration-response relationships of mutants of Asn 562 and d Tyr 563 measured in inside-out patches. a, c, d Information present averages of a number of experiments derived from impartial cells (WT n = 10, F518H n = 5, F518Q n = 4, F518Y n = 5-6, F518A n = 5-9, F518I n = 7, N562A, n = 5, N562L n = 4, Y563A n = 5, Y563H n = 3–6), errors are s.e.m., traces present match to a Hill equation. e, f Lipid transport of Phe 518 mutants quantified in a mobile scrambling assay. e Preliminary values recorded at resting Ca2+ focus and f ranges measured 600 s after utility of ionomycin, which will increase intracellular Ca2+. Particular person experiments are depicted as spheres (mock n = 10, F518I, F518A, F518Y n = 3, F518Q n = 4, F518H n = 6), errors are s.e.m. g, h Liposome-based in vitro scrambling assay of the reconstituted mutant F518H compared to WT. g Time-dependent fluorescence lower upon addition of the lowering agent dithionite (t = 60 s) at 0 and 1000 µM Ca2+ in comparison with WT. Information present imply of three technical replicates, errors (s.e.m.) are smaller than the displayed line width. h Ca2+-concentration response relationship of scrambling of the mutant F518H in comparison with WT obtained from three technical replicates (displayed in Supp. Fig. second). Values have been obtained as described within the strategies. Strong line exhibits match to a Hill equation, errors are s.e.m. Supply information are offered as a Supply Information file.
Constructions of activating mutants
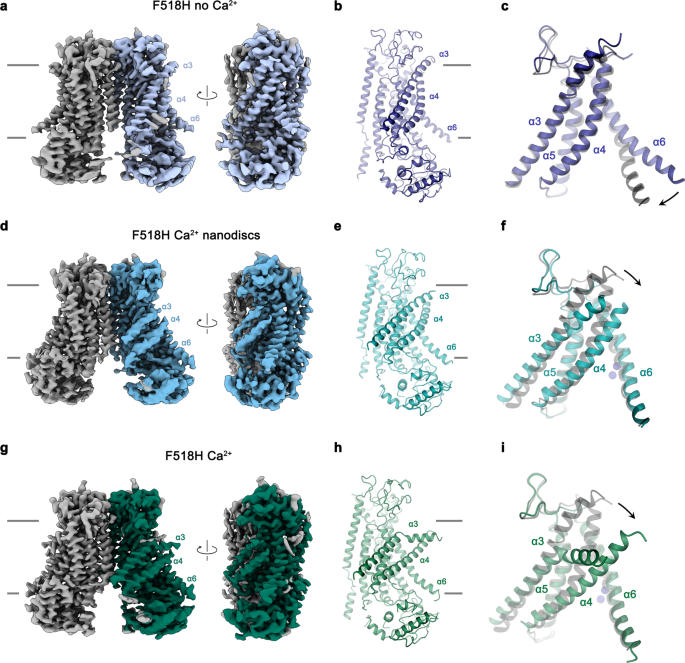
Within the subsequent step, we took benefit of the identification of activating mutants to seize conformational adjustments in TMEM16F that escaped detection in earlier buildings, as a result of excessive stability of closed states underneath the investigated circumstances. We thus got down to research the mutant F518H by cryo-electron microscopy (cryo-EM) and decided buildings of the Ca2+-free protein in detergent and the Ca2+-bound protein in each detergent and lipid nanodiscs (Fig. 3, Supplementary Figs. 3–5, Desk 1). All buildings are of top quality and have offered detailed perception into accessible conformations of TMEM16F. The construction of F518H in detergent in absence of Ca2+ (F518HnoCa) decided at 3.39 Å defines a protein conformation that’s similar to the corresponding construction of WT (WTnoCa, PDBID 6QPB, RMSD 0.64 Å)19 (Fig. 3a, b, Supplementary Figs. 3 and 6a). In comparison with the beforehand decided Ca2+-bound WT construction (WTCa, PDBID 6QP6)19, the intracellular a part of α6 has indifferent from the vacant Ca2+-binding web site by a rigid-body rotation round Gly 615 serving as a pivot for the displacement (leading to an RMSD of two.04 Å) (Fig. 3a–c, Supplementary Fig. 6a, b). Within the three buildings depicting presumable inactive states (i. e., WTnoCa, WTCa, and F516HnoCa), α3 and α4 are tightly packed in opposition to the core of the protein and engaged in quite a few interactions thereby sealing the assumed ion and lipid permeation path from the setting (Fig. 3a–c).
a Cryo-EM density of the F518HnoCa construction at 3.39 Å with one subunit proven in shade. b Ribbon illustration of the F518HnoCa subunit and c blowup of the pore area. d Cryo-EM density of the F518HCaND construction at 2.93 Å with one subunit proven in shade. e Ribbon illustration of the F518HCaND subunit and f blowup of the pore area. g Cryo-EM density of the F518HCa construction at 2.96 Å with one subunit proven in shade. h Ribbon illustration of the F518HCa subunit and i blowup of the pore area. a, d, g Relationships between views are indicated. a, b, d, e, g, h Traces point out membrane boundaries. c, f, i WTCa (PDBID 6QP6) is proven in grey for comparability, arrows point out actions. a–i Chosen transmembrane helices are labeled. f, i Ca2+ ions are displayed as blue spheres.
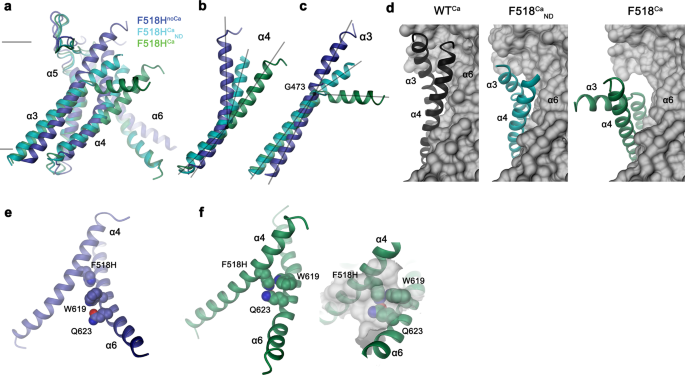
In distinction to F518HnoCa, the 2 buildings of the Ca2+-bound mutant, decided at 2.96 Å in detergent in presence of the lipid PIP2 (F518HCa, Supplementary Fig. 4, Desk 1) and at 2.93 Å in lipid nanodiscs (F518HCaND, Supplementary Fig. 5, Desk 1), undertake conformations which are distinct from WTCa (RMSDs 2.0 Å for F518HCaND and a pair of.88 Å for F518HCa) with each buildings carrying traits of an activated state (Fig. 3d–i, Supplementary Fig. 6b). Essentially the most pronounced variations in comparison with WTCa concern α-helices 3 and 4 and to a lesser extent α6 and its previous loop area (Fig. 3f, i, Supplementary Fig. 6b). On this transition, the F518HCaND construction exhibits an obvious intermediate in direction of the construction of the mutant in detergent since its coordinates are about midway on a possible trajectory from F518HnoCa to F518HCa (Fig. 4a–c). In F518HCaND, the poor decision of the α3-α4 pair in comparison with the remainder of the protein displays its excessive mobility underneath the investigated circumstances, whereas in F518HCa each helices are present in a single well-defined conformation (Fig. 3d, g, Supplementary Figs. 5, 6). In each ligand-occupied mutant buildings, the extracellular a part of the α3-α4 pair has indifferent from the remainder of the protein, thereby unleashing its tight interactions with α5 and α6 (Fig. 3f, i and 4d). In case of α4, this results in the straightening of the bent conformation adopted in WTCa by 16° in F518HCaND and 26° in F518HCa (Fig. 4b). In each buildings, the relief of the bent conformation of α4 is accompanied by a tilt of the helix and a shift by 3 Å alongside its axis in direction of the cytoplasm (Fig. 4a, b). The concomitant conformational change of α3 from WTCa to F518HCaND could be approximated by comparable rearrangements. The lean round an axis situated within the heart of the helix parallel to the membrane results in an outward motion of α3 by 25° at its extracellular and 10° at its intracellular half and a shift alongside its axis in direction of the cytoplasm (Fig. 4c). In comparison with F518HCaND, the intracellular a part of α3 in F518HCa is preserved, however there’s a further massive conformational change in proximity of Gly 473, the place the helix has unwound and its extracellular half has rotated as inflexible unit by 46° in direction of the membrane core to imagine a conformation that’s stabilized by contacts with the straightened α4 (Figs. 3i and 4a). As a consequence of the described transitions, the interplay interface between α4 and α6 decreases from 747 Å2 in WTCa to 535 Å2 in F518HCaND and 241 Å2 in F518HCa. Within the latter construction, the remaining contacts contain interactions of the mutated His 518 on α4 with Trp 619 and Gln 623 on α6, that are distant from one another within the WT construction (Fig. 4e, f). The described conformational adjustments outcome within the opening of a hydrophilic pore on the interface of α helices 3–6 with a diameter of 5 Å at its constriction, which might be of adequate dimension to allow conduction of ions which have stripped a big a part of their hydration shell (Fig. 5a–c, Supplementary Fig. 6c, d). The identical transition exposes polar residues on the higher a part of the subunit cavity to the membrane, which have been coated within the core of the protein within the apo construction of the mutant and apo and Ca2+-bound buildings of WT (Fig. 5d, e, Supplementary Fig. 6d). Though we can’t exclude the likelihood that in a completely scrambling-competent conformation, residual contacts between α4 and α6 could be damaged to open a subunit cavity that exposes its inside to lipids for your entire thickness of the membrane, as noticed in fungal TMEM16 scramblases, we didn’t discover any proof for such conformation in our information. We thus suppose that the noticed Ca2+-occupied F518H buildings are able to catalyzing lipid permeation. On this case, lipids would throughout their transition throughout the membrane surmount the contact area between α-helices 4 and 6 exterior of the subunit cavity, as described as potential state of affairs in earlier research19,22.
Superposition of the pore area of various buildings of F518H encompassing α-helices 3-6 (a), α4 (b) and α3 (c). b, c Traces point out axes for various helix sections to approximate conformational adjustments. d Packing interactions of α3 and 4 (ribbon) with the rest of the protein (depicted as molecular floor) in numerous conformations of TMEM16F. Relationship between α4 and α6 in e, F518HnoCa and f, F518HCa with inset displaying blow-up of the contact area with sections of the molecular floor proven.
a Pore area noticed within the construction of F518HCa. The view is from the surface. Inset depicts blow-up of the identical area with sidechains of chosen pore-lining residues proven as sticks. Crimson circle marks pore heart, asterisk, the place of Gly 473. b Channel considered from throughout the membrane. The protein-enclosed aqueous pore and the molecular floor dealing with the lipid blayer of the identical area are indicated. c Pore diameter estimated by Gap65 mapped alongside the pore axis from the surface (prime) to the within (backside) for indicated buildings. b, c, * and # point out equal positions in each panels. The molecular floor of the pore area of WTCa (d) and F518HCa (e) considered from throughout the membrane illustrates the publicity of hydrophilic patches to the lipid bilayer in F518HCa upon activation. Asterisk marks equal positions. a, b, d, e The molecular floor is coloured in line with the properties of contacting residues (blue for fundamental, purple for acidic and inexperienced for polar residues).
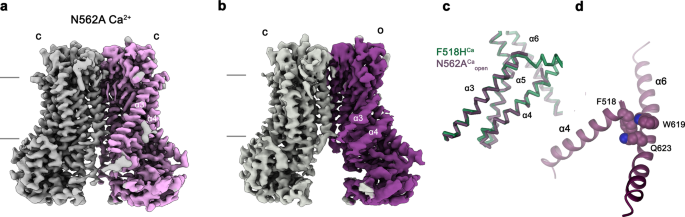
To analyze whether or not equal conformational adjustments, as discovered for the mutant F518H, would even be noticed in activating mutants which are situated distant from the α4-α6 interface, we’ve got decided the construction of the mutant N562A. This residue is situated on α5 and doesn’t line the expected pore. A dataset of the mutant in detergent, in presence of two mM Ca2+ and 0.01 mM of the lipid PIP2 (N562ACa), allowed a reconstruction of top quality, exhibiting two populations of the protein obtained after a 3D variability evaluation and refinement of the 2 finish states (Supplementary Fig. 7, Desk 1). One inhabitants, containing 62% of the labeled particles, shows C2 symmetry and was refined to three.01 Å (Supplementary Fig. 7c–f). It exhibits the protein in a non-conducting closed conformation (c) that’s primarily an identical to the construction of WTCa decided underneath equal circumstances (Fig. 6a). A second inhabitants encompassing 38% of the labeled particles, refined to three.49 Å, is uneven with one subunit residing in the identical non-conducting conformation noticed within the symmetric class and the opposite subunit exhibiting an association that intently resembles the construction of the mutant noticed within the dataset F518HCa (o), displaying related actions of α-helices 3, 4 and 6 (Fig. 6b–d, Supplementary Fig. 7c, g–j). As in F518HCa, α3 and α4 have rearranged in direction of the opening of the subunit cavity though the extracellular components of each α-helices are cellular and thus not outlined within the density (Fig. 6b–d, Supplementary Fig. 7i, j). Consequently, there isn’t a direct proof for a kinked conformation of α3 on this dataset. Assuming a binomial distribution of states in subunits that act independently within the dimeric protein, as described for the anion channel TMEM16A23,24, the noticed ratio means that the big predominance of the closed state in WT was perturbed within the mutant, the place 20% of subunits reside in an activated conformation. The construction of the N562A mutant thus confirms that the rearrangement of the subunit cavity in F518HCa and F518HCaND isn’t a consequence of the disturbed interface of α-helices 4 and 6, however as an alternative defines a structural transition in direction of the lively state that’s adopted upon Ca2+-binding. The absence of uneven conformations in datasets of F518H obtained underneath equal circumstances (Supplementary Figs. 4 and 5) additional emphasizes the gradual stabilization of the lively state, which is extra pronounced within the case of the mutant of the residue on α4 than noticed for N562A.
Cryo-EM densities of the symmetric closed dimer of N562ACa at 3.01 Å (a) and the uneven dimer at 3.49 Å with the activated subunit proven in shade (b). Subunit conformations are indicated as closed (c) and open (o). c Comparability of the open conformation of N562ACa and F518HCa. Pore-lining helices are proven as Cα-trace. d Relationship between α4 and α6 within the activated conformation of N562ACa. Residues which are in touch within the activated subunit are proven as space-filling fashions.
Options of the detergent and lipid layers surrounding TMEM16F
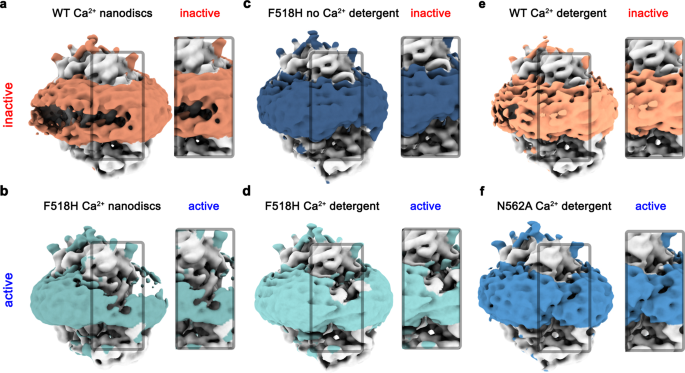
In all buildings decided on this research, options of the density surrounding the protein, which relying on the preparation correspond to both detergents or lipid nanodiscs, additional illustrate the impact of the noticed structural rearrangement on its setting. Whereas this density is common within the Ca2+-free conformation of F518H and round subunits in each populations of N562A the place the subunit cavity has remained closed, there’s a pronounced attenuation on the web site of conformational adjustments in subunits exhibiting an activated state in each detergent and lipid nanodiscs (Fig. 7). These anomalies within the density distribution level in direction of a destabilization of the lipid and detergent setting. As membrane thinning and bilayer distortion was beforehand thought-about as an indicator for lipid scrambling20,22,25,37, the affiliation of those options with the activated state means that the noticed rearrangements are related for the scrambling exercise of TMEM16F.
Densities of a Ca2+-bound TMEM16F WT in 2N2 lipid nanodiscs (PDBID 6QPC, WTCaND), b the Ca2+-bound TMEM16F F518H mutant in 2N2 lipid nanodiscs (F518HCaND), c the Ca2+-free TMEM16F F518H mutant in digitonin (F518HnoCa), d the Ca2+-bound TMEM16F F518H mutant in digitonin (F518HCa), e Ca2+-bound TMEM16F WT in digitonin (PDBID 6QP6, WTCa), and f the Ca2+-bound TMEM16F N562A mutant (N562ACa) within the uneven state considered in direction of the activated subunit. All maps have been low-pass filtered at 7 Å. The coloured area depicts the detergent micelle or nanodisc belt. a–f Insets present zoom into highlighted area. The view is in direction of the subunit cavity of 1 subunit. The practical state of the respective buildings (lively, inactive) is indicated.
Purposeful relationships between lively state mutants
Regardless of the described variations relating to conformational particulars, the buildings of the activating TMEM16F mutants F518H in detergent and lipid nanodiscs and N562A in detergent all present widespread rearrangements in direction of the opening of the subunit cavity within the Ca2+-bound state. These are characterised by the concerted motion of α-helices 3 and 4 away from the core of the protein that’s accompanied by a straightening of the bent α4 and its dissociation from α6 (Fig. 3f, i and 4a–d). To additional examine the relevance of the noticed conformational adjustments for activation, we’ve got studied combos of level mutants of concerned residues by patch-clamp electrophysiology and mobile scrambling assays.
One of many hanging options of F518HCa considerations a conformational change within the extracellular a part of α3, ensuing from its native unwinding near Gly 473, a residue that’s conserved in TMEM16A and F (Fig. 3h, i, 4c and 8a). We constructed mutants of this versatile residue with the purpose to rigidify this area and investigated the consequence of the mutation on activation. The mutation of the equal place (i.e., Gly 510) in TMEM16A to a inflexible proline, investigated in a earlier research, has proven a pronounced right-shift of the EC50 of Ca2+, reflecting a robust destabilization of the open state42. The concomitant weakening of its inhibition by an open-channel blocker, which binds to a close-by pocket that’s fashioned within the lively state, additional underlines the restriction of conformational rearrangements by the mutation42. The equal substitution of Gly 473 in TMEM16F to proline (G473P) exerts an much more pronounced impact and didn’t present any seen exercise in mobile scrambling assays and patch-clamp recordings, regardless of its concentrating on to the plasma membrane (Fig. 8b–d, Supplementary Fig. 8a). In contrast, a mutation of the identical residue to alanine (G473A) had no detectable influence on its practical habits (Fig. 8b, Supplementary Fig 8a, b). These outcomes illustrate the significance of the described place for structural rearrangements in TMEM16F underlying its activation.
a Conformation of α3 across the hinge residue Gly 473 (circle) in buildings obtained for the mutant F518H. b Lipid transport exercise of Gly 473 mutants quantified in a mobile scrambling assay. Information exhibits fluorescence ranges normalized to WT, at elevated Ca2+, 600 s after utility of ionomycin. Dashed line signifies imply worth of WT. Bars present imply of six organic replicates (depicted as spheres), errors are s.e.m. c Present magnitudes of HEK293T cells expressing WT or G473P recorded in excised patches. Bars signify imply of particular person experiments (n = 8), errors are s.e.m. d Floor expression of G473P. Anti-myc Western blot corresponds to biotinylated constructs pulled-down from HEK293T cells expressing myc-tagged WT or G473P after floor biotinylation. Samples are derived from the identical experiment, gels and blots have been processed in parallel. e Interactions between residues on α-helices 4 and 6 which are in touch within the lively however not the inactive state of TMEM16F. f Coupling energies between residues depicted in e. Inset exhibits scheme of the double-mutant cycle evaluation. WT/F518A/Q623A and WT/F518A/W619A confer with the cycle quantifying the impact of mutations F518A and both Q623A or W619A relative to WT, F518H/F518A/F518H_Q623A to the cycle of F518A and F518H_Q623A relative to the mutant F518H. g Contact area between α-helices 4 and 6 within the activated state of the double-mutant F518A_Q623ACa. Blow up exhibits residues which are in touch as space-filling fashions. h Comparability of the open conformation of the lively subunits of N562ACa and F518A_Q623ACa. Pore-lining helices are proven as Cα-trace.
The second pronounced characteristic noticed in all activated conformations considerations the rearrangement of α4 resulting in its dissociation from α6 and the formation of novel interactions between each helices. These contain contacts between the residue at place 518 with Trp 619 and Gln 623 (Fig. 4e, f). The latter residues are 6 and 11 Å aside from His 518 in F518HnoCa, precluding a direct interplay within the absence of Ca2+ (Fig. 4e, and 8e). To characterize the relevance of the noticed interactions for the activation course of, we’ve got investigated their energetic coupling in mutant cycles. In comparison with WT, the Ca2+ concentration-responses of respective alanine mutants of Phe 518 and Gln 623 are left-shifted, whereas the response of Trp 619 is nearly unchanged (Fig. 1a, Supplementary Figs. 1b and 8c, d). Equally, the double mutants of residue pairs on α4 and α6 are strongly left-shifted (Supplementary Fig. 8d). The crosstalk between residue pairs is mirrored in a weak coupling noticed upon the conversion of the EC50 values to energies, emphasizing a practical interplay within the WT protein (Fig. 8f, Supplementary Fig. 8e). Conversely, we questioned to which diploma the noticed proximity of the polar aspect chains of His 518 and Gln 623 within the mutant F518H would stabilize the noticed conformation. The pronounced coupling power of 1.4 kJ mol−1 obtained in a double mutant cycle within the background of the strongly left-shifted F518H illustrates a robust interplay between each residues within the lively state of the mutant protein (Fig. 8f, Supplementary Fig. 8e). To probe whether or not this interplay would lure the protein in an intermediate state by stopping an extra separation of α-helices 4 and 6 to completely open the subunit cavity, we’ve got decided the construction of the double mutant F518A/Q623A by cryo-EM (Supplementary Fig. 9, Supplementary Desk 1). On this double mutant, the truncation of each aspect chains precludes an equal polar interplay as noticed in F518H and would thus allow a rest of a doubtlessly strained conformation. Nevertheless, the construction of this double mutant doesn’t resemble the open cavity of nhTMEM16 and as an alternative exhibits very related properties as noticed within the mutant N562A, with two predominant channel populations (Supplementary Fig. 9). One in every of these populations is symmetric with each subunits residing in a Ca2+-bound inactive state, the opposite is uneven with one subunit exhibiting an inactive and the opposite an activated state (Supplementary Fig. 10a–c). Their related dimension signifies an open likelihood of about 30%, which is considerably larger than noticed for N562A. The conformation of the activated subunit strongly resembles its equal in N562A, aside from a small motion of α4 ensuing from the removing of two cumbersome aspect chains, and there’s no trace of an extra opening of the cavity (Supplementary Fig. 10d, e), which gives one other piece of proof for conformational variations within the activated conformations of TMEM16F and nhTMEM16.
Collectively, the outcomes obtained from the rigidification of a conserved glycine residue and the investigation of the brand new interplay area noticed within the Ca2+-bound, however not within the Ca2+-free state of activating mutants, underline the relevance of the decided buildings to explain important options of TMEM16F activation.