Cation-dependent maturation of CgMCA-I
To decipher the molecular determinants of the maturation strategy of CgMCA-I, the full-length (residues 1–392) wild-type enzyme was overexpressed in E. coli and purified by affinity chromatography and gel filtration. Measurement exclusion chromatography signifies that, the elution quantity of CgMCA-I corresponds to an obvious mass of 38 kDa with an extra peak at 10 kDa suggesting that the protein may very well be submitted to a maturation course of as noticed for different metacaspases14,15,16 (Supplementary Figs. 1, 2). It has been proven that calcium might facilitate and, in some circumstances, is obligatory12,14,16,17 for maturation of the enzyme. With a view to achieve a greater understanding of CgMCA-I maturation, we purified by affinity chromatography utilizing Biosprint 96 (see Maturation Assay, SI) and analysed by gel electrophoresis the wild-type protein after 0, 4 and eight days, respectively, within the presence or the absence of calcium or EGTA to look at the maturation course of over time and the influence of Ca2+ ions on this course of (Fig. 1).
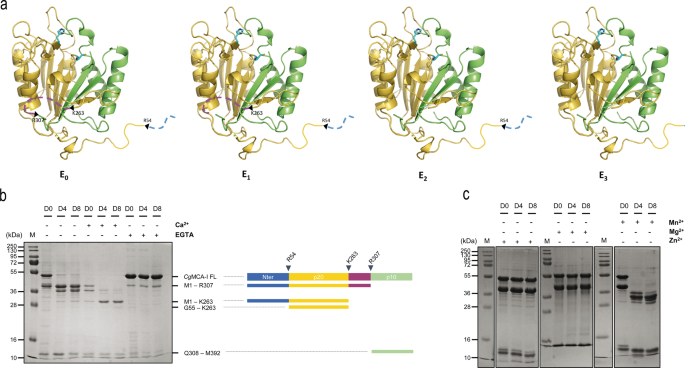
a Structural illustration of maturation intermediate types of CgMCA-I ranging from the non-matured kind (E0), after the primary and second cleavage (E1 and E2 respectively), and the mature kind (E3). b SDS-PAGE of CgMCA-I aliquots at maturation day 0 (D0), 4 (D4) or 8 (D8) within the presence or absence of Ca2+ (10 mM) or EGTA (1 mM). The band at 48 kDa corresponds to CgMCA-I full size (CgMCA-I FL). Schematic illustration of the maturation strategy of CgMCA-I with the N-terminal half, the p20 area, the linker, and the p10 area, respectively coloured in blue, yellow, purple, and inexperienced. The proteolytic cleavage after residues R307, K263 and R54 ends in the progressive look of maturation intermediate types which correspond to totally different bands recognized by their residues on the correct as decided by mass spectrometry. c SDS-PAGE of CgMCA-I aliquots at D0, D4, and D8 within the presence or the absence of Mn2+ (10 mM), Mg2+ (10 mM), and Zn2+ (10 mM).
Within the absence of calcium or EGTA, CgMCA-I undergoes cleavage and begins to maturate at day 0, persevering with till day 8 with out reaching an entire mature kind particularly the E3 kind (vide infra). Completely different middleman bands seem over time with sizes from 10 kDa to 38 kDa. The maturation within the presence of Ca2+ is clearly accelerated and at day 8, a skinny band at 23 kDa is seen which may very well be attributed to the p20 area. In the presence of EGTA, the method is stopped at day 0, confirming the necessity for a divalent cation within the maturation course of. Along with the full-length metacaspase at 48 kDa, a thinner band is noticed at 38 kDa indicating that maturation may begin throughout expression and purification previous to EGTA addition (Fig. 1b).
The truth that maturation is seen within the absence of extra Ca2+ after purification may point out that calcium partially binds to the protein throughout expression or that one other divalent cation may very well be current, even at low focus, and may very well be sure previous to purification. To substantiate the position of calcium and/or different divalent cations as activators, we examined three extra divalent metallic ions: Mn2+, Mg2+, and Zn2+ (Fig. 1c). Within the presence of Mn2+, the enzyme reveals a habits just like that noticed for maturation within the absence of divalent cations. Extra surprisingly, within the presence of Mg2+ or Zn2+, the maturation course of appears to be blocked on the very first cleavage occasion corresponding to within the presence of EGTA suggesting that these two divalent cations may play an inhibiting position on the maturation (Fig. 1c).
Evaluation of amino-acid residues concerned in CgMCA-I maturation
Evaluation of metacaspase fragments by nano-reversed section liquid chromatography coupled to high-resolution mass spectrometry (nanoLC-HRMS) with an Orbitrap analyzer was used to find out the cleavage websites with larger accuracy. The excessive decision of the orbitrap mass spectrometer permits figuring out, after deconvolution of the multicharged MS spectrum, the molecular weight of the totally different cleaved fragments of CgMCA-I with excessive accuracy (Supplementary Fig. 3). We recognized residues Arg307, Lys263, and Arg54, as the primary, second and third cleavage websites, respectively (Fig. 1b).
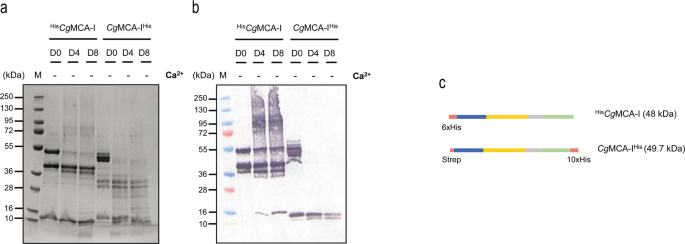
With a view to decipher the molecular determinants of the maturation course of, a sequence of cloning and maturation experiments had been carried out, and subsequently the time-course maturation with out components or within the presence of calcium or EGTA was explored. The primary goal was to find out the preliminary cleavage website. For this goal, CgMCA-I used to be cloned in a pET52 vector to introduce a His-Tag on the C-terminal (CgMCA-IHis) after which noticed following 0, 4, and eight maturation days with out components by gel electrophoresis and (Fig. 2a) by western blotting (Fig. 2b). A band round 10 kDa seems at day 0 for CgMCA-IHis however solely at day 4 for HisCgMCA-I, indicating that the primary cleavage happens on the C-terminal a part of the protein. As well as, the shortage of band at 49.7 kDa at day 4 for CgMCA-IHis signifies that the discharge of the C-terminal happens within the very first steps of maturation. However, the presence of a band at 48 kDa within the western blot till day 8 means that the cleavage of the N-terminal a part of the protein happens on the very finish of the method (Fig. 2a–c). To substantiate this statement, the R54A mutant was expressed and its maturation analyzed (Supplementary Fig. 4a). It seems that within the presence of calcium the method is delayed, main solely to intermediate types of maturation with out reaching a completely mature type of CgMCA-I after eight days, and confirming that cleavage at place R54 is most certainly the final one to happen.
With a view to characterize the second and first cleavage websites which needs to be at positions 263 and 307, respectively, Lys263 and Arg307 had been mutated to an alanine (K263A and R307A) and the maturation course of adopted (Supplementary Fig. 4b, c). Surprisingly, each mutants appear to endure cleavages with a maturation course of mimicking the wild-type enzyme, therefore Lys263 was mutated to an aspartate and to a phenylalanine, respectively, to induce extra drastic results (Supplementary Fig. 4d, e). In each circumstances, and within the lack of calcium, the primary cleavage occasion was noticed at day 0 with the looks of a band at 38 kDa. Nevertheless, in the midst of maturation within the days following, an aberrant course of appears to happen with quite a few secondary cleavage websites ensuing within the disappearance of the protein from day 4. Within the presence of calcium, solely a skinny band with a measurement which may correspond to the mature kind was noticed. This implies that the mutants likely underwent aberrant maturation, probably partially inflicting their degradation (Supplementary Fig. 4d, e). Altogether, this factors to the presence of fundamental residues not being obligatory at these positions, offered that the residue changing the lysine doesn’t drastically change the perform held by the facet chain.
General structural options of CgMCA-I
To elucidate the molecular determinants for cation-dependent metacaspase activation, CgMCA-I used to be expressed in E. coli and purified to homogeneity with out components or within the presence of 10 mM Ca2+ or of 1 mM EGTA with the intention to acquire non-maturated, partially or totally maturated enzyme for crystallization. Alternatively, we used a purified C238A catalytic mutant with the intention to acquire a non-maturated protein.
Crystallization assays had been carried utilizing enzymes at day 0 and day 4 following purification. Relying on the time of maturation, crystals grew both from an answer containing 0.2 M (NH4)2SO4, 30% (w/v) PEG 4000 (day 0 protein) or an answer containing 0.2 M magnesium acetate, 20% (w/v) PEG 3350 (day 4 protein).
Constructions from these two crystal types had been decided by the molecular alternative technique utilizing the construction of the homologue from Saccharomyces cerevisiae, ScMCA-I (PDB code: 4F6O16), as search mannequin. The crystal constructions displayed clear electron density for Ca2+ and Mg2+ ions, and had been named in accordance with this concern, CgMCA-ICa (PDB code: 7QP1) or CgMCA-IMg (PDB code: 7QP0), respectively (Fig. 3).
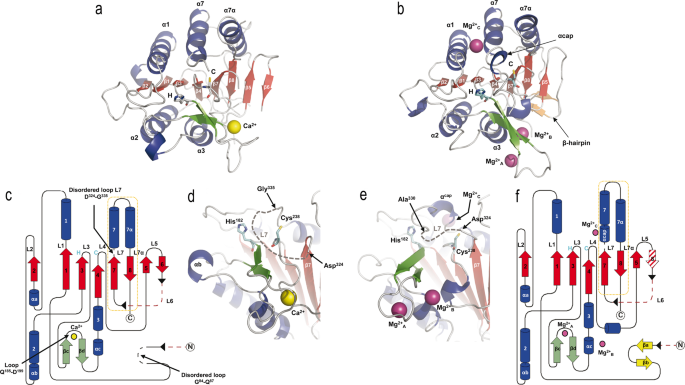
Three-dimensional constructions of CgMCA-ICa and CgMCA-IMg monomers, respectively, in advanced with (a) Ca2+ (yellow sphere) or (b) Mg2+ (purple spheres). α-Helices (blue) and β-strands (pink), with the numbering of the secondary construction components proven in white and black, respectively (a). The three Mg2+ ions current in CgMCA-IMg are named Mg2+A, Mg2+B, and Mg2+C (b). Catalytic residues are highlighted as stick displays by H (His182) and C (Cys238) and coloured in cyan. Topological illustration of CgMCA-ICa (c) and CgMCA-IMg (f). Disordered components of the proteins are proven in black dotted traces and lacking components of the protein as a result of maturation course of are proven in pink dotted traces (c, f). Cleavage websites are indicated by black arrow heads and the p10 subunits are framed by an orange dotted line (c, f). Loops numbering (L1 to L7/L7α) are indicated (c, f). Catalytic website group of CgMCA-ICa (d) and CgMCA-IMg (e), displaying the place of the 2 catalytic residues (His182 and Cys238) respective to the cations binding websites and the disordered L7 loop, for which the disordered half is indicated by grey dotted traces.
CgMCA-ICa crystallized within the trigonal house group P32 with two molecules within the uneven unit (Desk 1, Supplementary Fig. 5a), whereas CgMCA-IMg crystallized within the monoclinic house group C2 with two molecules within the uneven unit (Desk 1, Supplementary Fig. 5b). The constructions had been solved to respectively 3.0 Å and 1.6 Å decision and share widespread options. The monomers CgMCA-ICa and CgMCA-IMg each comprise a p20 and a p10 area forming a caspase-like core by which a two-stranded anti-parallel β-sheet from the p10 area completes a six stranded parallel β-sheet within the p20 area (Fig. 3a–c, f). This central β-sheet is surrounded by three helices on one facet (α1, α4, and α5) and two α-helices on the opposite facet (α2 and α3) (Fig. 3a–c, f). As well as, a β-hairpin (βc and βd) between β3 and β4 full the p20 area (Fig. 3c, f). Cys238 and His182 are positioned on loops CgL4 and CgL3, respectively, from the catalytic dyad (Fig. 3a–c, f).
Concerning the specificities of CgMCA-ICa, three quick helices (αa, αb and αc) full the p20 area (Fig. 3a, c). As well as, as noticed within the crystal construction of ScMCA-I16, loop CgL7 (named L3 in ScMCA-I), which can cap the substrate-binding groove, is disordered from Asp324 to Gly335 (Fig. 3c, d, Supplementary Fig. 6). Versus ScMCA-I, a brief loop fashioned by residues Gly84 to Gln87, shows no electron density (Fig. 3c). Surprisingly, the 2 monomers of the CgMCA-ICa dimer noticed within the uneven unit work together by way of a β-sheet complementation between the β6 and β5’ strands (Supplementary Fig. 5a). This sudden group being just like that of the dimer normally noticed for caspases, SAXS experiments had been carried out to find out the oligomeric state of CgMCA-I within the presence of calcium and confirmed that this enzyme is monomeric in resolution (Supplementary Fig. 7).
Concerning the maturation course of, the N-terminal and the linker positioned between p20 and p10 domains and which corresponds to the CgL6 loop, are each cleaved and residues Met1 to Gly68 and Asn260 to Ile309 present no electron density within the 2Fo-Fc map (Fig. 3c, Supplementary Fig. 8a, b). Because of the flexibility on the cleaved ends, we weren’t capable of decide with accuracy the residues after which the cleavage occurred solely based mostly on the construction, nevertheless, the boundaries of the lacking half are in excellent accordance with the cleavage website recognized by mass spectrometry (Supplementary Fig. 3).
Concerning the specificities CgMCA-IMg, and versus CgMCA-ICa, no β-strand complementation was current to stabilize the dimer which as a substitute reveals contacts between helix 1 and loop CgL2 (Supplementary Fig. 5b). The general construction is extremely just like that of CgMCA-ICa with a RMSD of 0.823 Å calculated on all Cα atoms. As for CgMCA-ICa, a completely maturated protein was noticed, however with the N-terminal (Met1 to Ser72) and linker (Asn260 to Ile309—part of CgL6) lacking (Fig. 3f, Supplementary Fig. 8c, d). Versus CgMCA-ICa, residues Gly84 to Gln87 present clear electron density and kind a hairpin stabilized by two quick β-strands (βa and βb) (Fig. 3b, f, Supplementary Fig. 8e, f). A disordered CgL7 loop remains to be noticed, however the phase from Asp330 to Gly335 may very well be constructed. Apparently, this longer structured phase types a one-turn helix (Gly335-Asn337, αcap) and caps the substrate-binding groove limiting, beneath this conformation, the entry to the catalytic website (Fig. 3e).
Structural foundation for CgMCA-I Ca2+ dependent maturation and activation
A number of research have proven that the maturation strategy of metacaspases (AtMCA-IIb17, AtMCA-IId12, TbMCA-Ib14, ScMCA-I16) is calcium-dependent. To resolve the construction of TbMCA-Ib14, the authors used samarium, a calcium-mimicking lanthanide. They reported that the presence of Ca2+ at all times hindered crystal formation and that Ca2+ couldn’t be detected after the soaking experiment. Extra not too long ago, a three-dimensional construction of a plant metacaspase15 was solved and a soaking experiment carried out within the presence of Ca2+. Right here, once more and regardless of clear conformational reorganization of some components of the protein, no electron density was noticed for Ca2+.
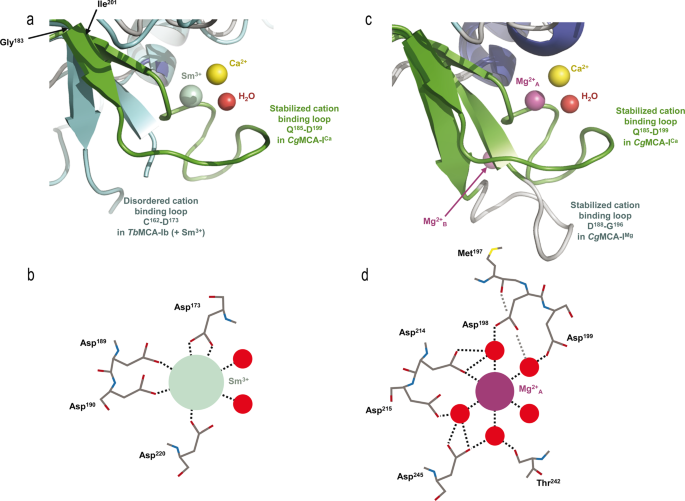
To find out the construction of CgMCA-ICa, we purified the wild-type metacaspase within the presence of 10 mM CaCl2 and carried out crystallization assays at day 0 utilizing a non-fully maturated protein. Crystals had been noticed solely after three months, and the crystal construction reveals that the maturation course of occurred in the midst of the crystallization. We noticed a transparent electron density for Ca2+ (Figs. 3a, d, 4, Supplementary Fig. 9). Apparently, the Ca2+ ion is within the neighborhood of the Sm3+ binding website as noticed in TbMCA-Ib. In CgMCA-ICa (Fig. 5a), Ca2+ is hepta-coordinated by a water molecule and residues Gly196, Asp198 (Asp173 in TbMCA-Ib), Asp214 (Asp189 in TbMCA-Ib), Thr242 and Asp245 (Asp220 in TbMCA-Ib) (Supplementary Fig. 5a, b). Equally, Sm3+ in TbMCA-Ib is hepta-coordinated however reveals a distinct binding mode14. Certainly, aside from the three conserved aspartates (Asp173, Asp189 and Asp220), the remainder of the coordination is ensured by two water molecules and Asp190 (Fig. 5b). As well as, Arg171 and Ser217 in TbMCA-Ib which correspond to Gly196 and Thr242 in CgMCA-ICa, respectively, are usually not concerned within the cation binding (Fig. 5b). Altogether, this authentic Ca2+ coordination ends in an entire reorganization of phase Gly183-Ile201 and its related loop Gln185-Asp199 (Fig. 5a).
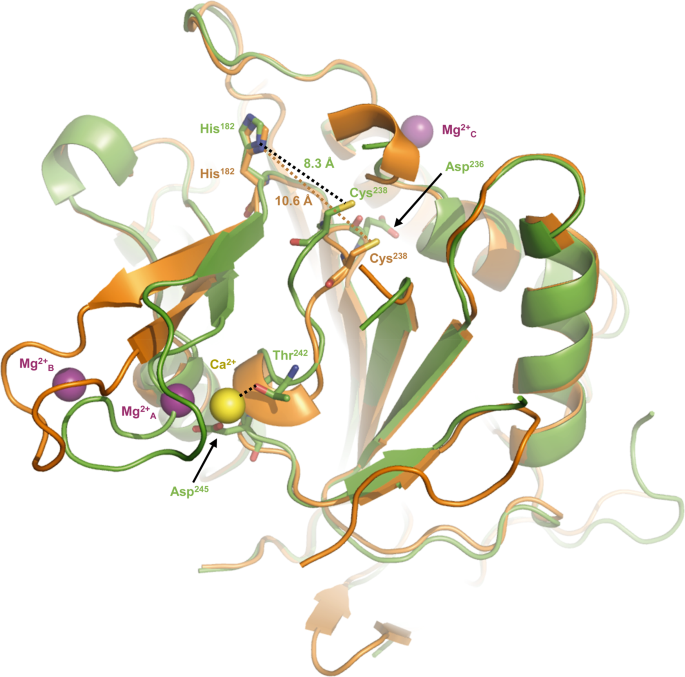
The space between the thiol group of the catalytic cysteine Cys238 and the imidazole nitrogen of the catalytic histidine His182 within the two crystal constructions, with that of CgMCA-ICa proven in inexperienced and CgMCA-IMg in orange. Ca2+ and Mg2+ ions are proven as yellow and purple spheres, respectively.
a, c. Positions of Ca2+ in yellow and the coordinating water molecule in pink (CgMCA-ICa) respective to Sm3+ in inexperienced (TbMCA-Ib, PDB code: 4AFP) (a) and Mg2+A in purple (CgMCA-IMg) (b). Loop Gln185-Asp199 surrounding the Ca2+ (CgMCA-ICa) is proven in inexperienced (a, c), the disordered loop Cys162-Asp173 in TbMCA-Ib is proven in cyan (a) and loop Asp188-Gly196 in CgMCA-IMg is proven in grey (c). Schematic illustration of the coordinated Sm3+ (b) and Mg2+A (d) as noticed within the constructions of TbMCA-Ib and CgMCA-IMg, respectively.
First, Ca2+ is barely displaced (3.4 Å) in comparison with Sm3+ (Fig. 5a). Second, a TbMCA-Ib disordered loop (Cys162-Asp173) is stabilized in CgMCA-ICa (loop Gln185-Asp199) as a result of coordination by Gly196 and Asp198 (Supplementary Fig. 10a, b). This loop conformation reveals clear electron density by which the calcium is capped. Importantly, this loop is a part of a small area manufactured from two small antiparallel β-strands and one quick helix, a website which instantly succeeds the loop CgL3 bearing the catalytic histidine (Fig. 3c). Third, the phase Asp236-Asp245 (Asp211-Asp220 in TbMCA-Ib) which holds the catalytic cysteine is stabilized by Thr242 and Asp245 (Fig. 4, Supplementary Fig. 10b). Apparently, the “cation binding loop” noticed in ScMCA-I (Gln223-Asp237) and in TbMCA-Ib (Cys162-Asp173) is ordered in CgMCA-ICa which means that the calcium has in all probability a job of stabilization not just for loop Asn185-Asp199 but additionally for phase Asp236– Asp245, the entire contributing to bringing collectively the 2 catalytic residues (Fig. 4).
To resolve the construction of CgMCA-IMg, we carried out experiments just like these described above. However, after purification, the enzyme maturated throughout 4 days previous to crystallization assays. For the crystals used for construction willpower, the crystallization situation included 200 mM magnesium. The electron density map revealed the presence of three magnesium ions being hexa-coordinated (Fig. 5c, d, Supplementary Fig. 10c–e). One of many Mg2+ ions is positioned at the exact same place because the samarium ion noticed within the crystal construction of TbMCA-Ib however as a substitute of being coordinated by 4 amino-acid residues and a water molecule as noticed for this latter, in CgMCA-IMg it’s coordinated by 5 water molecules and Asp214 (Asp189 in TbMCA-Ib) (Fig. 5d). Apparently, many of the aspartates coordinating samarium in TbMCA-Ib are conserved in CgMCA-IMg and work together with three of the water molecules coordinating magnesium, particularly Asp198 (Asp173 in TbMCA-Ib), Asp215 (Asp190 in TbMCA-Ib) and Asp245 (Asp220 in TbMCA-Ib). As well as, Asp199 and the primary chains of Thr242 and Met197 work together with the 2 final water molecules (Fig. 5b, d). Altogether, it seems that Sm3+ likely mimics Mg2+ slightly than Ca2+. The truth that samarium is noticed in non-maturated TbMCA-Ib may clarify the totally different orientations noticed for the cation binding loops of the 2 enzymes (Fig. 5a, c). In CgMCA-IMg, the enzyme is totally maturated however in parallel, we’ve got proven that Mg2+ appears to impair the maturation of the metacaspase. Apparently, within the presence of Mg2+ the space between the 2 catalytic residues is 10.6 Å, in opposition to 8.3 Å when in presence of Ca2+, suggesting that the presence of calcium forces the catalytic website to undertake a correct group for its autolysis exercise (Fig. 4). A second magnesium ion coordinated by two acidic residues (particularly Asp188 and Asp190) and three water molecules is current within the “calcium capping loop” contributing to its stabilization and reorientation (Supplementary Fig. 10c, e).
Enzymatic evaluation of CgMCA-I maturation
In preliminary experiments, obvious kinetic constants of CgMCA-I had been decided within the presence of 10 mM calcium at days 0 and day 8 of maturation utilizing Z-GGR-AMC as substrate (focus vary of 0–50 µM) (Supplementary Fig. 11). A kinetic mannequin based mostly on the enzymatic steady-state hydrolysis of Z-GGR-AMC together with chemical hydrolysis was used to find out kinetic parameters (Supplementary Desk 5).
The primary-order kinetic fixed for the non-catalyzed (okchem) response is comprised between 0.1183+/− 0.0092 min−1 and 0.107+/− 0.012 min−1. As anticipated, the enzymatic response follows Michaelis-Menten behaviour within the substrate focus vary assayed. At day 0, the okcat worth is 2.8+/− 0.7 min−1 and reaches 15.8+/− 0.6 min−1 at day 8 indicating that the matured metacaspase is 5 to six occasions extra lively than the non-matured kind. Apparently, the Okm worth for Z-GGR-AMC (18 µM) doesn’t change throughout maturation, which may clarify why the substrate binding website is barely barely affected. This implies that the change in okcat is slightly as a result of native spatial reorganisation of the catalytic website residues. These observations are supported by the afore talked about crystal constructions of CgMCA-ICa and CgMCA-IMg which highlighted that the presence of calcium narrows the space between the 2 catalytic residues stabilizing in the identical time this conformation (Fig. 4).
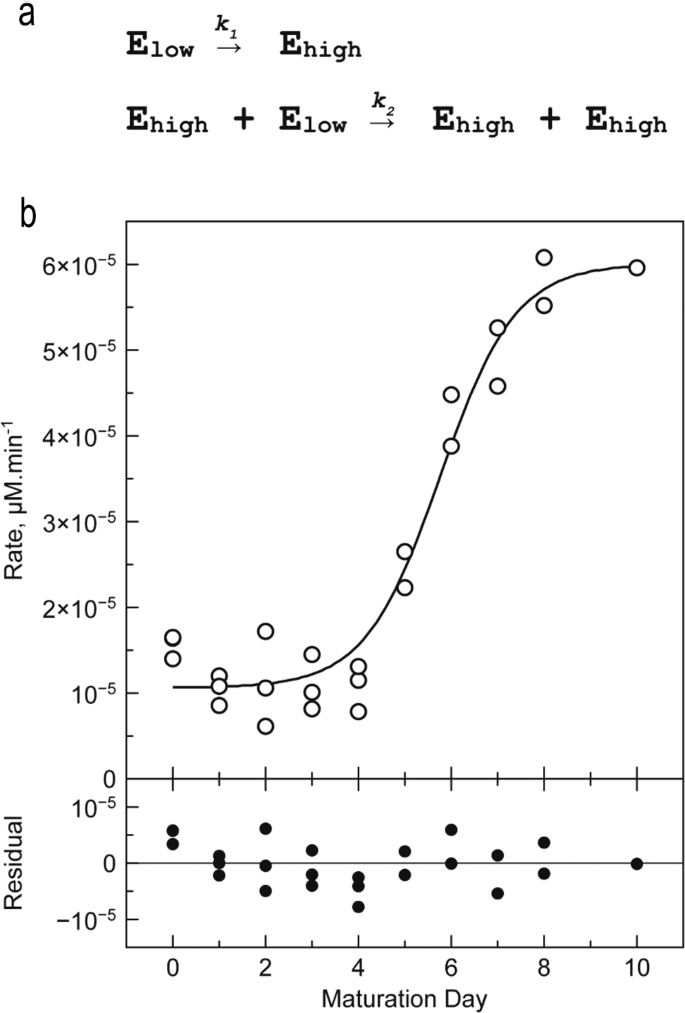
With a view to comply with the maturation course of, purified enzyme was saved at 22 °C within the absence of calcium and exercise was decided day by day within the presence of 10 mM Ca2+ over 10 days (Fig. 6). The exercise curve is a sigmoid that may be interpreted as follows.
a Two-step mannequin describing the transition of enzymes of the Elow inhabitants into enzymes of the Eexcessive inhabitants. b Hydrolytic exercise decided throughout a 10-days maturation experiment. CgMCA-I (16.5 µM) is saved at 22 °C in 10 mM HEPES buffer pH 7.6, 150 mM NaCl. Response circumstances: [CgMCA-I] = 2 µM, [Z-GGR-AMC] = 50 µM, 10 mM HEPES buffer pH 7.6, 150 mM NaCl, 10 mM CaCl2 (n = 3 impartial experiments).
From days 0 to 4, the exercise is barely larger than the background sign (~10.10−6 µM min−1). The primary metacaspase types E0, E1, E2 recognized by gel electrophoresis on the early stage of maturation, are usually not totally catalytically lively (Fig. 1). At day 5, the exercise will increase sharply reaching a most of ~60.10−6 µM min−1 at day 8. That is according to the 6-fold improve of okcat noticed within the earlier experiment. Of those maturation steps, CgMCA-I kind E3 recognized by gel electrophoresis seems to be the totally lively enzyme state. After day 8, all enzyme molecules are principally of their catalytically lively (mature) kind.
From an exercise standpoint, the sigmoid form of this curve means that two enzyme populations exist: a primary inhabitants (particularly Elow) with lowest particular exercise comprising E0, E1, and E2 states; and a second inhabitants (particularly Eexcessive) with the best particular exercise composed by the E3 state. In each populations, the excellence between enzymes of the identical exercise is inconceivable.
The sigmoid form of the curve suggests a trans mechanism, i.e. enzymes of the Eexcessive inhabitants are capable of maturate enzyme of the Elow inhabitants to afford Eexcessive enzymes. A number of fashions had been evaluated to explain the kinetic mechanism of the transition from the Elow to the Eexcessive inhabitants (Supplementary Tables 5, 6). Essentially the most acceptable mannequin is a two-step mannequin the place the primary transition from the Elow to the Eexcessive inhabitants follows a cis mechanism, whereas the second step is a trans mechanism by which enzymes of the Eexcessive inhabitants catalyse the maturation of the Elow enzymes into Eexcessive enzymes inhabitants, just like an autocatalytic mechanism (Fig. 6a). With a view to validate this speculation, we combined a completely maturated enzyme with a catalytic mutant CgMCA-IC238A/H182A, unable to maturate (Supplementary Fig. 12b). We noticed after a interval of 8 days that the catalytic mutant was certainly maturated by the lively enzyme current at low focus.
Obvious kinetic constants of step one are about 600 occasions decrease than these of the second step (Supplementary Desk 8), thereby explaining why, beneath our experimental circumstances, the looks of the enzyme with excessive exercise follows practically an OFF-ON behaviour.
When the response is carried out within the presence of EGTA (1 mM) or within the absence of calcium, no vital exercise is noticed throughout and after eight days evidencing that calcium is required for the exercise. With a view to assess the affinity of CgMCA-I for Ca2+ at totally different levels of maturation, we carried out nano-DSF experiments and measured the intrinsic fluorescence of CgMCA-I within the absence or presence of Ca2+ after totally different incubation occasions (0, 1 h, or 24 h) (Supplementary Fig. 13). For CgMCA-I, the obvious Okd is barely growing with incubation time, indicating that even the unmatured type of the enzyme is ready to bind Ca2+ (Supplementary Fig. 13b). Regarding, CgMCA-I at day 8 of maturation, the obvious Okd doesn’t change drastically with incubation time and in comparison with CgMCA-I at maturation day 0 with 24 h of Ca2+ incubation (Supplementary Fig. 13). Altogether, this might point out that the elevated exercise from the Elow types to the Eexcessive kind can be as a result of maturation of the enzyme (successive cleavages) slightly than to an elevated calcium affinity.
Concerning the cleavage website mutants, all of them show totally different habits from a catalytic standpoint (Supplementary Fig. 14). Mutants K263A, K263F, K263W, and R307A all confirmed a delay within the activation course of. Moreover, K263F and K263W are much less lively after eight days of maturation. For mutant R307A, a speedy activation at day 4 however a decreased exercise at day 8 was noticed, illustrating an aberrant maturation. Mutant R54A confirmed essentially the most intriguing habits with a better exercise than that of the wild-type enzyme all through the maturation course of, and will counsel that the N-terminal, when un-mutated, may modulate the metacaspase exercise previous to cleavage within the final step of the maturation. These outcomes additionally confirmed that the presence of fundamental residues on the totally different cleavage websites, not less than in vitro, are usually not obligatory to mature the protein, and that the cleavage might be extra structure-dependent. Nevertheless, the anarchic ranges of exercise obtained for the totally different mutants counsel {that a} correctly matured metacaspase requires the presence of those residues.