Abstract: Opioid withdrawal cuts off the availability of serotonin to the nucleus accumbens, dramatically lowering sociability in mice and growing social aversion.
Supply: Stanford
The acute bodily sickness characterizing opioid withdrawal is hard sufficient to endure even with full household, neighborhood, and medical help—so it’s a brutal and typically lethal irony that one among withdrawal’s salient signs is excessive social aversion.
“Self-isolation could cause addicted folks to drop out of restoration applications, to get into conflicts, and to tug away from household and different social help networks that would assist them to stay abstinent,” says Stanford psychiatry professor Keith Humphreys, Ph.D., a global skilled on habit therapy and public coverage.
New analysis by the lab of Stanford neuroscientist Robert Malenka, MD, Ph.D. has recognized a key molecular hyperlink between opioid withdrawal and social aversion within the brains of mice—suggesting the potential to assist folks in restoration from opioid habit reconnect with their social help networks.
The research factors to a key function for the neuromodulator serotonin in switching sociability on and off. Usually, Malenka says, sociability is determined by launch of serotonin in an space deep within the mind referred to as the nucleus accumbens, which usually performs a key function in linking behaviors to motivation and reward.
Of their new research, revealed October 5, 2022, within the journal Neuron, the researchers found how opioid withdrawal cuts off the availability of serotonin to this area, dramatically lowering sociability.
Malenka’s group performed the research on a mouse mannequin of opioid withdrawal. The researchers gave escalating doses of morphine to the mice till the rodents have been addicted after which abruptly reduce off the drug. They then examined the mice for sociability.
“We measured how a lot time a mouse needs to spend hanging out with just a little buddy. It’s actually that straightforward,” says Malenka, the Nancy Pritzker Professor of psychiatry and behavioral sciences and deputy director of the Wu Tsai Neurosciences Institute.
“Throughout protracted withdrawal from opioids, each single mouse within the research had extreme sociability deficits.”
Malenka and his group had beforehand documented different sturdy hyperlinks between sociability and serotonin launch within the nucleus accumbens. In 2018, his group confirmed that lowered serotonin within the nucleus accumbens may account for social deficits in a mouse mannequin of autism.
Restoring serotonin launch largely restored typical ranges of rodent sociability. A yr later, they discovered that massive boosts of serotonin in the identical mind area “explains the extraordinary pro-social results of MDMA,” Malenka says. “We suspect the identical factor is occurring in people.”
These earlier research led Malenka and his colleagues to surprise if one thing related was occurring throughout withdrawal from opioids. “Maybe, just like the autism mouse mannequin, topics aren’t getting the traditional launch of serotonin that’s required for a pro-social, adaptive, non-aggressive interplay—so that they grow to be socially avoidant and cranky,” he says.
Malenka lab postdoctoral scholar Matthew Pomrenze, who led the group’s newly revealed research, made the connection {that a} explicit sort of opioid receptor molecule is thought to cut back serotonin launch inside the nucleus accumbens. This receptor subtype, referred to as a kappa receptor, had been linked to emphasize, melancholy and asocial conduct, however precisely the way it influenced sociability was unknown.
Pomrenze and colleagues carried out detailed experiments which revealed that in opioid withdrawal, a neuropeptide molecule referred to as dynorphin is launched within the nucleus accumbens, the place it prompts kappa receptors and blocks serotonin launch.
“We thought possibly that is what’s inflicting the asocial conduct we see in withdrawal,” Malenka says. “So, we requested—if we gave the animals a drug that blocks this kappa receptor and restores serotonin launch, may it additionally restore regular ranges of sociability?”
To make the experiment extra clinically related, Malenka’s group used a kappa receptor blocker referred to as aticaprant, which is already being examined for the therapy of sure subtypes of melancholy in people. When Malenka’s group gave the drug to their withdrawn, addicted mice, “it fully reversed their sociability deficits,” says Malenka.
Reaching one thing related for people may have a profound affect on the opioid epidemic, says Humphreys, who co-directs the NeuroChoice Initiative with Malenka and psychology professor Brian Knutsen, however was not concerned within the present research.
If opioid-addicted folks going by means of withdrawal turned towards sources of help slightly than away from them, many extra would possibly efficiently climate the discomforts of withdrawal and attain restoration with the assistance of buddies, household, their docs, and applications, he says.
“Withdrawal is usually the time when a physician says, ‘You don’t should dwell like this. We’ve got wonderful therapies that I’d be completely satisfied to attach you to.’ Or when somebody the addicted particular person is aware of says, ‘I used to be hooked on heroin for 10 years, and I’m not utilizing anymore. I’d be completely satisfied to take you to my Narcotics Nameless assembly.’ But when the particular person in withdrawal is avoiding all social interactions, none of these doubtlessly life-saving interventions can happen,” says Humphreys.
“That is precisely what NeuroChoice was designed to do,” says Humphreys. “To discover throughout ranges, from the cell to the person, to the group, to coverage. And from animals to people.”
However creating medicine to assist with habit is notoriously tough, Malenka cautions. For one factor, pharmaceutical corporations don’t reap large earnings from such medicines. “So, the objective of our sort of labor,” Malenka says, “is to persuade these within the human scientific analysis world to say, “Hey, that is working very well in mice, I ought to strive it in a scientific research.’”
The mechanism revealed by Malenka’s group’s research will give whoever could try this a superb head begin.
About this opioid withdrawal and social neuroscience analysis information
Creator: Gordy Slack
Supply: Stanford
Contact: Gordy Slack – Stanford
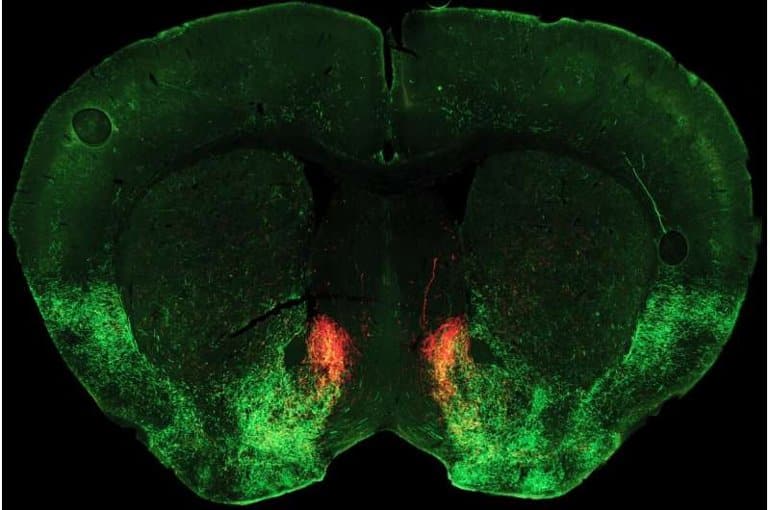
Picture: The picture is credited to Matthew Pomrenze, Malenka Lab
Unique Analysis: Closed entry.
“Modulation of 5-HT launch by dynorphin mediates social deficits throughout opioid withdrawal” by Matthew B. Pomrenze et al. Neuron
Summary
Modulation of 5-HT launch by dynorphin mediates social deficits throughout opioid withdrawal
Highlights
- Protracted opioid withdrawal results in sturdy social interplay deficits in mice
- Kappa opioid receptor activation within the NAc is critical for withdrawal social deficits
- Dynorphin-producing neurons within the DR promote withdrawal social deficits
- KORs scale back 5-HT launch within the NAc throughout withdrawal to mediate social deficits
Abstract
Social isolation throughout opioid withdrawal is a serious contributor to the present opioid habit disaster.
We discover that sociability deficits throughout protracted opioid withdrawal in mice require activation of kappa opioid receptors (KORs) within the nucleus accumbens (NAc) medial shell.
Blockade of launch from dynorphin (Pdyn)-expressing dorsal raphe neurons (DRPdyn), however not from NAcPdyn neurons, prevents these deficits in prosocial behaviors.
Conversely, optogenetic activation of DRPdyn neurons reproduced NAc KOR-dependent decreases in sociability. Deletion of KORs from serotonin (5-HT) neurons, however not from NAc neurons or dopamine (DA) neurons, prevented sociability deficits throughout withdrawal.
Lastly, measurements with the genetically encoded GRAB5-HT sensor revealed that in withdrawal KORs block the NAc 5-HT launch that usually happens throughout social interactions.
These outcomes outline a neuromodulatory mechanism that’s engaged throughout protracted opioid withdrawal to induce maladaptive deficits in prosocial behaviors, which in people contribute to relapse.

