Researchers from North Carolina State College and the College of Texas at Austin have outlined the construction of a substrate-bound iron 2-oxoglutarate (Fe/2OG) enzyme to discover whether or not these enzymes could possibly be used to create a big selection of molecules. They probed the enzyme’s lively website to find out its capability to bind with completely different substrates. Moreover, relatively than oxygen-addition, they noticed that Fe/2OG enzymes doubtless make the most of cations—extremely reactive species—to drive desaturation throughout catalysis. The work, revealed in Nature Communications, might result in using Fe/2OG enzymes in making a big selection of beneficial molecules.
Enzymes within the Fe/2OG household are naturally occurring—they’re present in all the pieces from micro organism to vegetation and animals. As such, these enzymes have the potential for use as a greener, extra environment friendly platform for creating molecules akin to vinyl isonitriles, which have antibiotic properties. Nonetheless, the pathways by which Fe/2OG enzymes create these molecules are poorly understood.
“The endgame is to know how the enzymes on this household create explicit molecules, in order that we will doubtlessly piggyback on a pure course of that present chemistry can not replicate,” says Wei-chen Chang, affiliate professor of chemistry at North Carolina State College and co-corresponding creator of a paper describing the work. “So we checked out a few completely different enzymes inside the Fe/2OG household to see how they carried out completely different transformations utilizing the identical substrate, or molecule they bind to.”
By specializing in how the enzyme binds to a selected substrate, the researchers can decide which different substrates could possibly be utilized by the enzyme, a extra environment friendly approach to decide potential reactions and merchandise than experimentation.
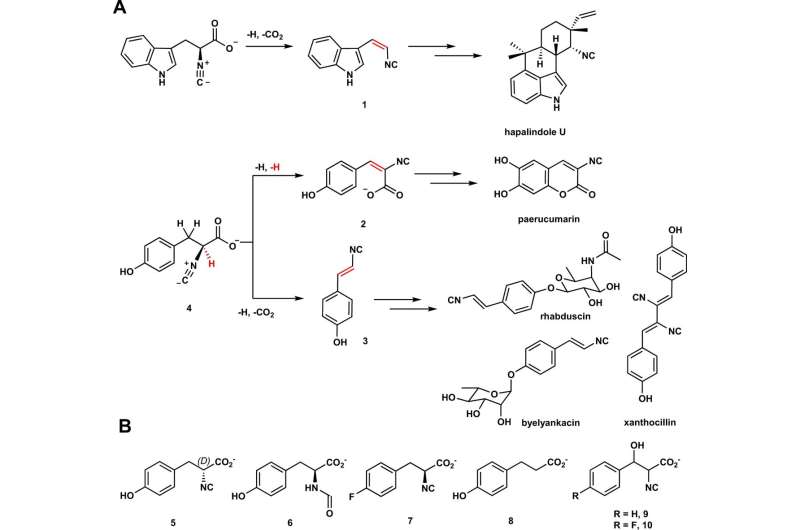
The analysis crew targeted on two Fe/2OG enzymes—PvcB and PlsnB—and in contrast their constructions and the merchandise they made. They recognized binding websites on each enzymes, however when it got here to exploring how the enzyme carried out its transformations, they made a shocking discover.
“Usually, the way in which Fe/2OG enzymes catalyze or create a brand new product occurs like this: the enzyme binds to the substrate, a single oxygen atom from molecular oxygen (O2) is launched into the substrate, and oxygen-addition drives the response,” Chang says. “That course of known as hydroxylation.
“However for these enzymes, the transformation or response is not pushed by hydroxylation, however by a reactive cation that triggers the following desaturations, the place new bonds are launched.”
The 2 Fe/2OG enzymes studied (PIsnB and PvcB) utilized basically distinct desaturation to create completely different merchandise from the identical substrate.
“Now we all know how these enzymes catalyze transformations and have discovered the binding websites, we have now a basis for figuring out what they will do by way of reactions,” Chang says. “We will additionally advocate and predict the perfect substrates to make use of to get focused merchandise.”
Wantae Kim et al, Elucidation of divergent desaturation pathways within the formation of vinyl isonitrile and isocyanoacrylate, Nature Communications (2022). DOI: 10.1038/s41467-022-32870-4
Quotation:
Researchers discover enzymes that use a cation, not oxygen-addition, to drive reactions (2022, September 12)
retrieved 12 September 2022
from https://phys.org/information/2022-09-explore-enzymes-cation-oxygen-addition-reactions.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.

