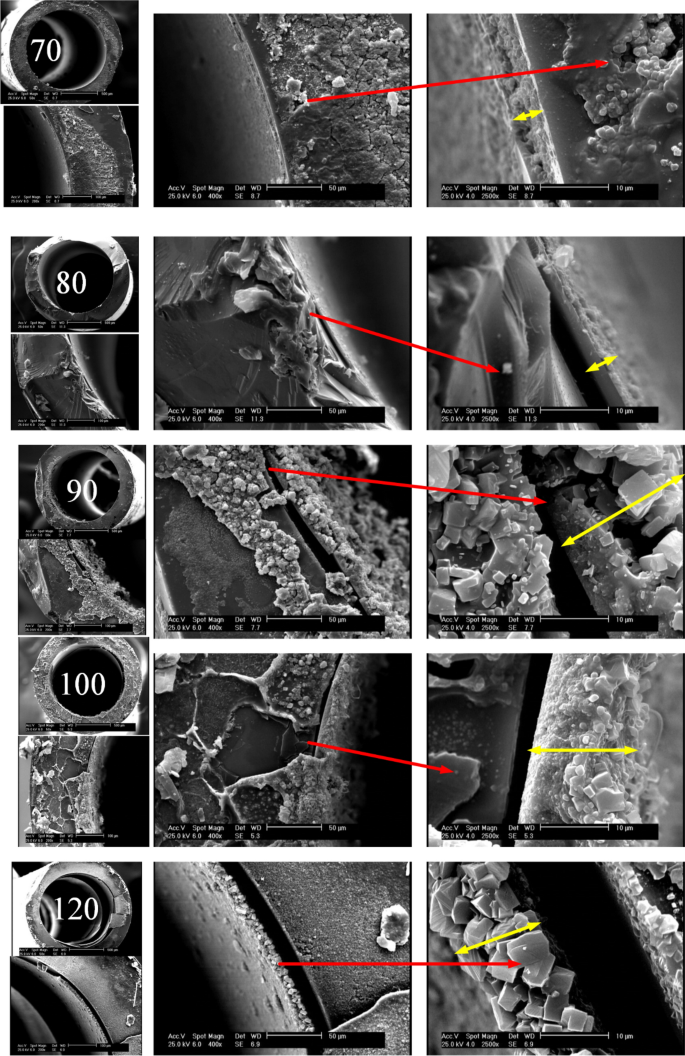
Cross-sectional view of TiO2 coated microchannels
To watch how temperature influences the thickness of the synthesized TiO2 layer, SEM snapshots of capillaries cross-sections at totally different scales together with 500, 100, 50 and 10 µm and numerous synthesis temperatures from 70 to 120 °C have been taken. The yellow arrows symbolize the thickness of the layer. In accordance with Fig. 2, at 70 and 80 °C, the TiO2 layer is extra uniform and the impact of temperature on the layer thickness is incremental. At 90 °C, particles of various sizes are fashioned, which causes the thickness of the layer to extend with a higher slope. At 100 °C, as a result of initiation of extra nucleation, extra particles are fashioned with smaller sizes. At 120 °C, particles with extra common shapes and comparatively shut sizes are fashioned, which might be as a result of balanced longitudinal and transverse progress of the TiO2 construction.
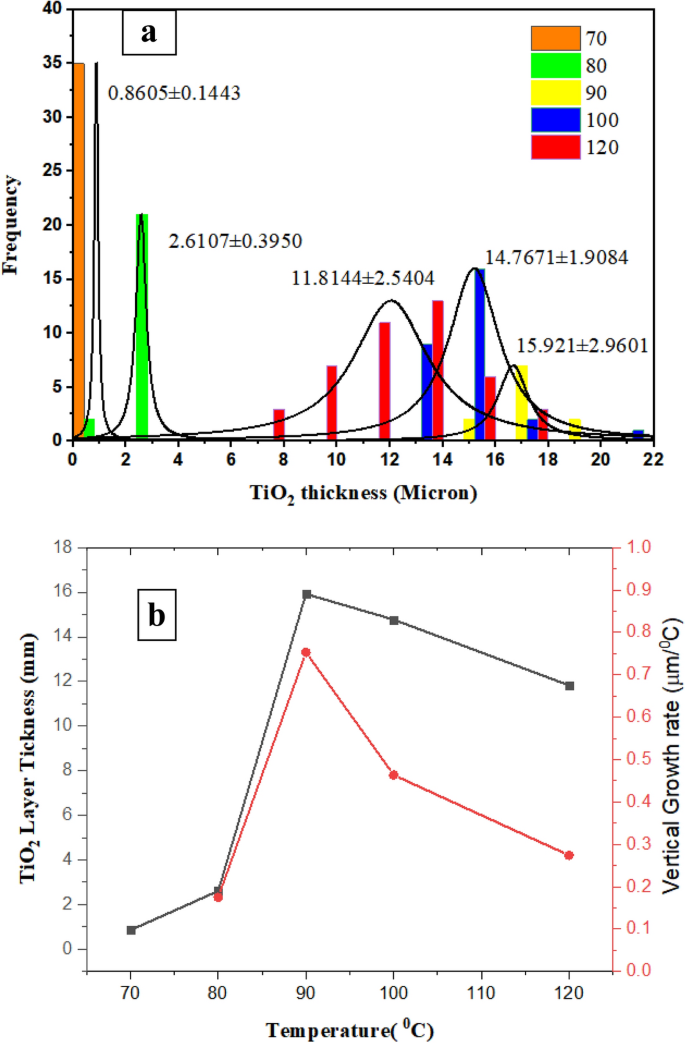
TiO2 progress fee and histogram
In accordance with Fig. 2 and with assist of picture j software program, the next histogram (Fig. 3a) is attained for TiO2 layer thickness, which exhibits the impact of temperature within the vary of 70–120 °C just isn’t inevitably ascending. In different phrases, from 70 to 90 °C, the layer thickness and its customary deviation improve. From 90 to 120 °C, the layer thickness decreases. At 90 °C, because of secondary nucleation, it’s a turning level in microfluidic synthesis. Such conduct was noticed within the bulk system at 180 °C. The best synthesis temperature might be 70 °C. First, TiO2 measurement is smaller, which is bigger in response to15 equivalent to the particular floor space. Second, measurement distribution is the narrowest.
Determine 3b exhibits a graph of TiO2 layer thickness improve and longitudinal progress. Development fee ((LT-L70)/∆T) is taken into account based mostly on the speed of the typical thickness improve at every temperature relative to the typical layer thickness at 70 °C divided by its temperature distinction with 70 °C.
As a result of improve in temperature from 70 to 80 °C, the layer thickness will increase from 860 to 2.61 mm. When the synthesis temperature will increase from 70 to 90 °C, the layer thickness reaches 15.92 µm, and the expansion fee will increase greater than 4 occasions. As soon as the synthesis temperature will increase from 70 to 100 °C, the layer thickness reaches 14.77 µm, and the expansion fee will increase greater than 2.5 occasions. With the synthesis temperature rising from 70 to 120 °C, the layer thickness reaches 11.82 µm, and the expansion fee will increase greater than 1.25 occasions.
Floor examine of the inside wall microchannel
In accordance with the SEM photos proven in Fig. 4, the synthesis at 70 °C resulted within the manufacturing of fifty nm particles which can be homogeneously dispersed on the floor. A couple of nanoclusters with a most diameter of 700 nm are additionally witnessed, however they’re much smaller in measurement and frequency in comparison with those detected at greater synthesis temperatures. Because the temperature will increase, quite a few secondary nuclei might be seen, which peaks at 90 °C. At 80 °C, the presence of a number of particles about 5 microns in size is seen, indicating a rise within the particle measurement distribution vary. At 90 °C, the variety of bigger particles is bigger. At 100 °C extra particles are fashioned with smaller sizes. At 120 °C excessive floor porosity and particle progress might be seen. EDX outcomes verify the uniformity of the coating and the presence of titanium dioxide.
Hurtado et al. 2016 proved that the photonic effectivity is 2 orders of magnitude greater within the coated capillary reactor than within the slurry stirred reactor (STR), and it’s twice slurry capillary16. It ought to be famous that an aqueous resolution is compelled by a micro-packed mattress capillary, it is going to are inclined to circumnavigate the beads as an alternative of passing by them, which impacts the yield of the method17.
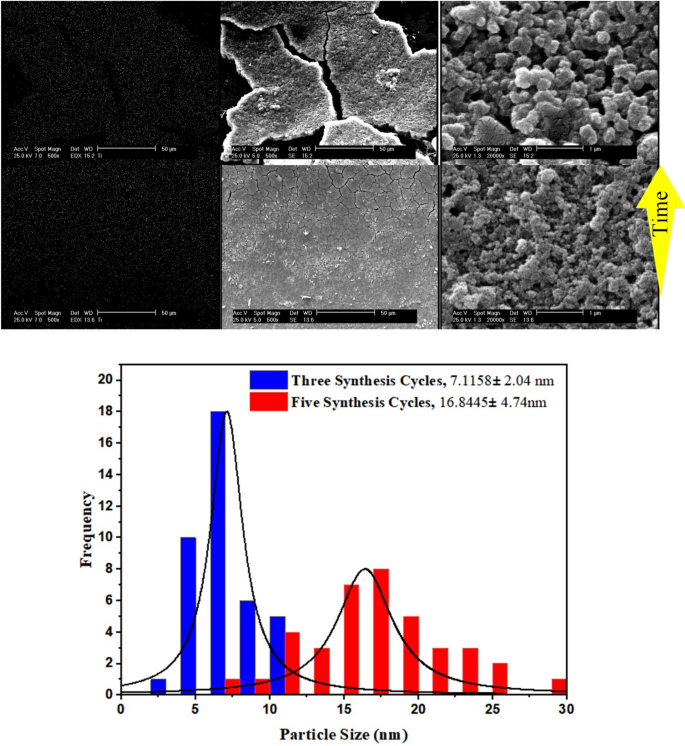
How synthesis cycles impacts TiO2 measurement
To research the impact of synthesis time on morphology, following the synthesis procedures, the one variable was the synthesis time. Due to this fact, the precursor options are injected into the capillaries for 3 and 5 consecutive cycles at 80 °C. A complete of 75 and 125 min had been dedicated to synthesis, after which the capillaries are transferred to a standard oven and heated at 140 °C for 1 h to make the coated TiO2 layer sturdy. As proven in Fig. 5, the particle measurement will increase with rising synthesis time, in addition to the cracks on the floor.
The histograms signifies that synthesis time of 75 min results in TiO2 imply measurement of seven.11 nm with 2.04 nm customary deviation. As soon as synthesis time will increase to its 1.67fold, each the particle measurement and measurement distribution proliferates greater than two occasions.
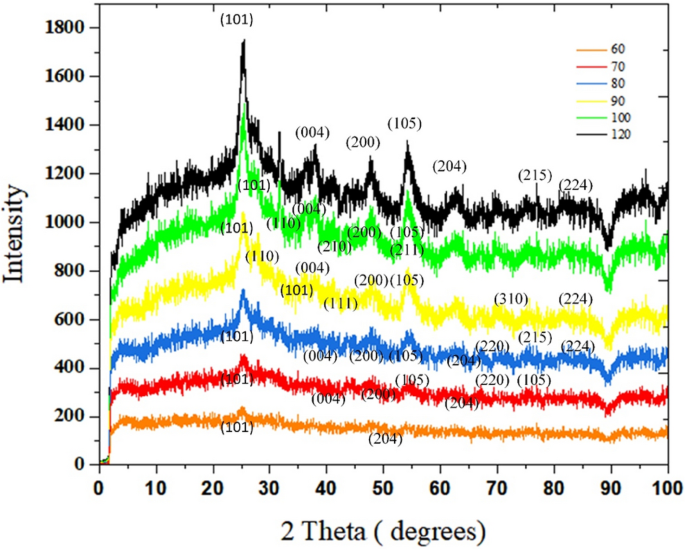
Crystallographic examine of TiO2 powder
The XRD spectrum of samples synthesized at totally different temperatures within the vary of 60–120 °C is given in Fig. 6. It’s famous that these samples are collected from the effluents of the capillaries. With synthesis temperature of 60 °C, two anatase peaks of (101) and (200) planes seem. Because the temperature rises to 80 °C, the sum of peaks proliferates. (7 peaks for 70 °C and eight peaks for 80 °C haven been detected). For 70 °C, Anatase peaks at 25.5°, 38.3°, 48.9°, 54.5°, 63.6°, 71.3°, and 76.3° are related to (101), (004), (200), (105), (204), (220), (215) crystalline planes18. Such peaks are additionally noticed for the pattern synthesized at 80 °C, as well as a brand new peak at 82.1° (224) (Anatase XRD JCPDS Card no. 78-2486).
Each phases of anatase and rutile are detected within the spectra of the synthesized samples at 90–100 °C. At 90, 5 anatase peaks that are associated to (101), (004), (200), (105), and (224) planes are seen, plus 4 peaks newly fashioned rutile peaks of 27.4°, 36.3°, 41.1°, 69.8°, which ascribes to (110), (101), (111), (310) 19,20. As soon as TiO2 is synthesized at 100 °C, in addition to (101), (004), (200), (105) crystalline anatase planes, three rutile peaks have been witnessed at 31.7° (110), 45.5° (210), 54.3° (211)20,21,22. Within the final spectra, which is said to the pattern synthesized at 120 °C, the depth of peaks has reached the best amount. Anatase peaks related to (101), (004), (200), (105), (204), (215), (224) crystalline anatase planes18,19.
XA (%) is the burden proportion of anatase the samples which consists of each anatase and rutile phases. It’s calculated from the next equation:
$${textual content{XA }} = frac{100}{{left( {1 + 0.26 * left( {IR / IA} proper)} proper) }}$$
(1)
the place “I” represents the utmost peak depth, A represents anatase and R denotes rutile. The burden proportion of rutile is obtained by subtracting the burden proportion of anatase from 100. In accordance with the XRD outcomes, from 60 to 80 °C, the anatase part is fashioned. Growing the synthesis temperature from 80 to 100 °C has led to partial conversion of anatase to rutile part. To calculate the crystallite measurement (D), the Scherer equation (Eq. 2) is used.
$$D=frac{Klambda }{beta COStheta }$$
(2)
the place Ok is the Scherer fixed (correction issue associated to the pattern form and is the same as 0.9, λ beam wavelength, β peak width at half the utmost peak, θ is the diffraction angle.
Common crystallite measurement (({D}_{ave})) might be resulted from Eq. (3) 23:
$${D}_{ave}={D}_{A}left(frac{{I}_{A}}{{I}_{A}+{I}_{R}}proper)+{D}_{R}left(frac{{I}_{R}}{{I}_{A}+{I}_{R}}proper)$$
(3)
Desk 1 exhibits that as synthesis temperature rise, crystallite measurement increments 24,25, however as soon as new crystalline part is being fashioned, a lower in common crystallite measurement is noticed. From 60 to 70 °C, anatase single crystallites develop. The onset of rutile part formation is 80 °C, the crystallite measurement will get a bit smaller. The crystal measurement diminished attributable to the burst of nucleation was extra palpable than that of the anatase crystal progress accelerated attributable to the rise of temperature26. At 90 °C, a substantial amount of anatase and rutile nucleons with a really small common crystallite measurement of 8.84 nm have been fashioned. Growing the temperature as much as 100 °C, the typical crystallite measurement has been doubled. Furthermore, in multiphase samples, by rising the rutile/anatase ratio (0.97–1.17) and rising the synthesis temperature (90–100 °C), the typical crystallite measurement will increase from 8.84 to 18.9 nm. An identical development is reported in27, the place lowering anatase/rutile ratio from 5.4 to 0.25, and synthesis temperature from 55 to 90 °C, the typical crystallites develop from 33.16 to 88.44 nm27. The synthesis temperature of 120 °C results in pure anatase nucleation and progress, and crystallite measurement reaches 88.68 nm.
DLS examine of TiO2 powder
To have an estimate of the scale of TiO2 powder, a sequence of dynamic gentle scattering checks have been carried out. For pattern preparation, a solvent is required, herein, isopropyl alcohol is used because the liquid medium with a refractive index of 1.36 and viscosity of 1.057 cP. The Pade Laplace algorithm is taken into account. The findings are summarized in Desk 2, because the synthesis temperature rises, the diameters of nanoparticles lower, and the diffusion coefficient will increase. The outcomes are in settlement with Figs. 3 and 4, which notify that near-wall TiO2 nanoparticles turn out to be greater with temperature improve, and due to this fact, the centered nanoparticles have a descending relationship with temperature. Likewise, with the immobilized TiO2, a sudden improve of measurement at 90 °C is noticed.
Identification of ZnO nanoparticles
In an effort to examine the outcomes of the microfluidic system with bulk, the molar ratio of ZnCl2 to NaOH is saved fixed at 0.6 with concentrations within the microfluidic system for the primary and second streams are 30 and 50 mM, respectively (50 occasions dilution of the majority programs). A microfluidic platform for synthesis comes together with extra management over response, within the meantime, the discount of chemical compounds consumption strongly prevents the incidence of chemical accidents. Particularly for extremely acidic or fundamental circumstances. Synthesis time in room temperature spiral microfluidic system has been carried out in 15 min V.S 2 h at 100 °C for the majority strategy.
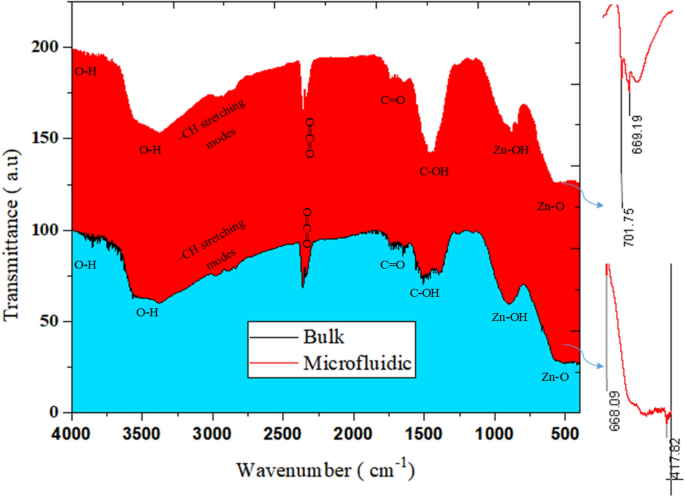
In accordance with Fig. 7, the outcomes of FTIR evaluation related to ZnO synthesis within the bulk and microfluidic system state the existence of an identical peaks within the two samples. ZnO stretching vibrations are noticed for each strategies within the vary of 400–668 cm−128. Within the vary of 870–1000 cm−1, there are Zn–OH peaks which can be greater in quantity and depth for nanostructures synthesized by the microfluidic methodology. Within the vary of 1200–1500 cm−1, the C–OH bond, which might be fashioned on account of the OH group because of washing with alcohol, signifies the bending vibration inside –OH group in plain29,30. These peaks are seen extra within the pattern of the majority methodology. Carboxylic group (C=O) was noticed round 1700 cm−1 31,32. A peak might be detected at 2300 cm−1, which signifies the atmospheric absorption of CO233. Within the vary of 2900–2800 cm−1, it exhibits the peaks associated to uneven and symmetric stretches of –CH and –CH2 teams. Peaks attributed to OH group stretching vibration have been recognized at wave numbers greater than 3600 cm−1. The presence of O–H stretches and hydrogen bonding by alcohol or water molecules might be present in peaks at 3400 or 3300 cm−1 34,35.
The impact of precursors flowrate on ZnO measurement
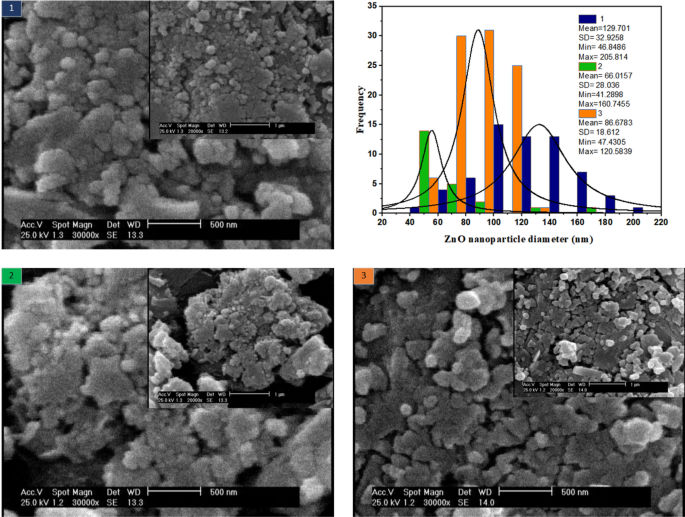
The intention of this part is to research the morphology of ZnO nanoparticles synthesized within the spiral system at room temperature. For the reason that mixing in this kind of microreactor is ultrafast, speedy nucleation is predicted. An easy approach is to tune the movement fee which influences the interface concentrations. Therein, the impact of the volumetric movement fee of ZnCl2 and NaOH streams are thought-about in three circumstances (1) each: 25 µl/min (2) each: 50 µl/min (3) zinc chloride: 25 µl/min and sodium hydroxide movement: 50 µl/min.
SEM illustrations in Fig. 8 are supplied in two magnifications of 500 and 1 µm. Within the 1st case, the place the volumetric movement of the 2 streams is low and equal, the residence time is excessive, resulting in bigger particles and a broader ZnO measurement distribution. Within the 2nd case, when the movement fee doubles in comparison with the primary case, the nanoparticle measurement is sort of halved and the ZnO measurement distribution is considerably diminished. Within the third case, the place the ZnCl2 movement fee is the same as 25 µl/min and the NaOH movement fee is the same as 50 µl/min, the particle measurement is intermediate. As well as, the narrowest measurement distribution is said to this case.
Organic exercise of immobilized enzyme
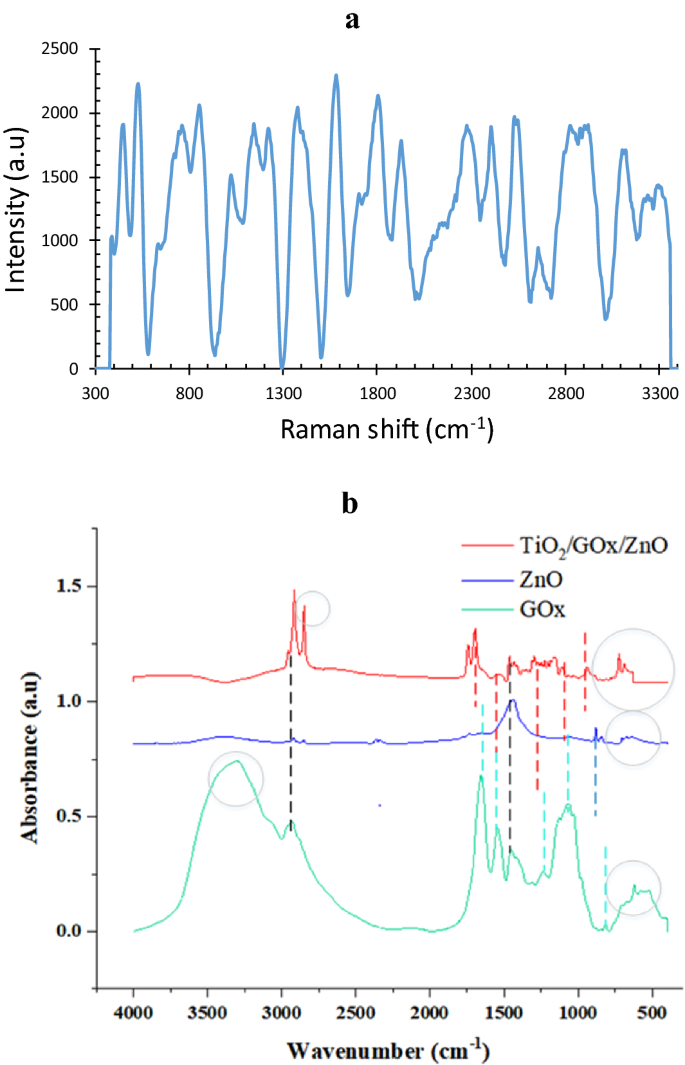
For example, Raman evaluation of bio-photo-catalyst with a GOx/ZnO ratio equal to 0.25 is proven in Fig. 9a some assignments of Raman vibrations modes of GOx are proven within the spectrum, that are in settlement with36. The outcomes evidently validate that the exceptional SERS exercise of the fabricated bio-photo-catalyst is induced by the synergistic results of the plasmonic Zn2+37, semiconductor TiO2, and glucose oxidase molecules, not solely induced by plasmonic metallic Zn nanoparticles. ZnO lattice vibration is related to a pointy peak at 454 cm−138. At 532 cm−1 corresponds to the oxygen vaccines within the TiO2/GOx/ZnO. The height at 856 cm−1 is equivalent to TiO239. Furthermore, the bioconjugation of ZnO/GOx 40 and the bandgap discount of TiO2 on account of doping with ZnO could have an effect on the Raman shift. Pure glutaraldehyde has a powerful band at 1125 cm−1 41 that originates from C−OH stretching. Nonetheless, crossed linked GOx confirmed a band at 1146 cm−1. This shifting might be defined by the conjugation of TiO2 and ZnO limiting the vibrations of oxygen.
The outcomes of FTIR evaluation for bio-photo-catalyst elements are proven in Fig. 9b. Within the ZnO pattern, ZnO tensile vibrations had been decided with peaks of 701 and 669 cm-128. Within the TiO2/GOx/ZnO pattern, a peak at 668 cm−1 may correspond to Ti–O–Ti and ZnO42. Two peaks within the vary of 900–800 cm−1 consult with Zn–OH29,30. Peaks 939, 1101, 1159, 1206, 1226, 1297 cm−1 are associated to Zn-OH and Ti–OH42. The presence of this bond signifies hydrolysis of the precursor 43. For glucose oxidase, a peak at 1062 cm−1 was noticed for the stretching vibrations of the C–O bond. Within the meantime, this peak with a decreased depth is detectable within the bio-photo-catalyst Peaks within the enzyme within the vary of 1300–1400 cm−1 may also belong to the phenolic group of glucose oxidase44. Within the bio-photo-catalyst spectrum, the height on this area can be noticed.
Two varieties of peaks had been noticed in all three samples. The presence of peaks within the vary of 1400–1500 cm−1 could also be associated to the (CH)n bonds within the fatty acid or enzyme. Furthermore, the C−H stretching modes of α-carbon and the aliphatic carbon chain of glutaraldehyde might be assigned close to 2800 cm−1 41,45,46.
Within the spectrum associated to glucose oxidase enzyme, the presence of peaks associated to amides of the primary, second, and third varieties of peptide construction can be noticed. At 1242 cm−1 and 1244, that are associated to C–N and C–H stress and N–H torsion47. Receptor bonds of the primary sort had been noticed at 1600 cm-1 to 1700, that are associated to the tensile vibration of C=O or CO peptide bonds within the protein construction, second amide bonds at 1500–1600 cm−1, NH stress, and C–N stress of the peptide teams. Major amines are generally used to watch structural adjustments in proteins, and this exhibits the organic exercise of GOx48,49. These peaks coexist within the bio-photo-catalyst, nonetheless, in comparison with pure glucose oxidase, their depth is diminished as a result of bioconjugation. There’s additionally a peak at 1743 cm−1 and 1699 which belongs to the carbonyl group50. As well as, GOx exhibits amide-related bonds at 3400–3440 cm−1 and amide B at about 2900 cm−1, which originate from the Fermi resonance between the primary peak of amide II and the N–H tensile vibration51. The peaks noticed within the two samples ZnO and GOx within the vary of 3300 cm−1 point out the presence of floor water and O–H bond42,46. This peak confirms the presence of hydrophilic spots31,32.
Optical examine
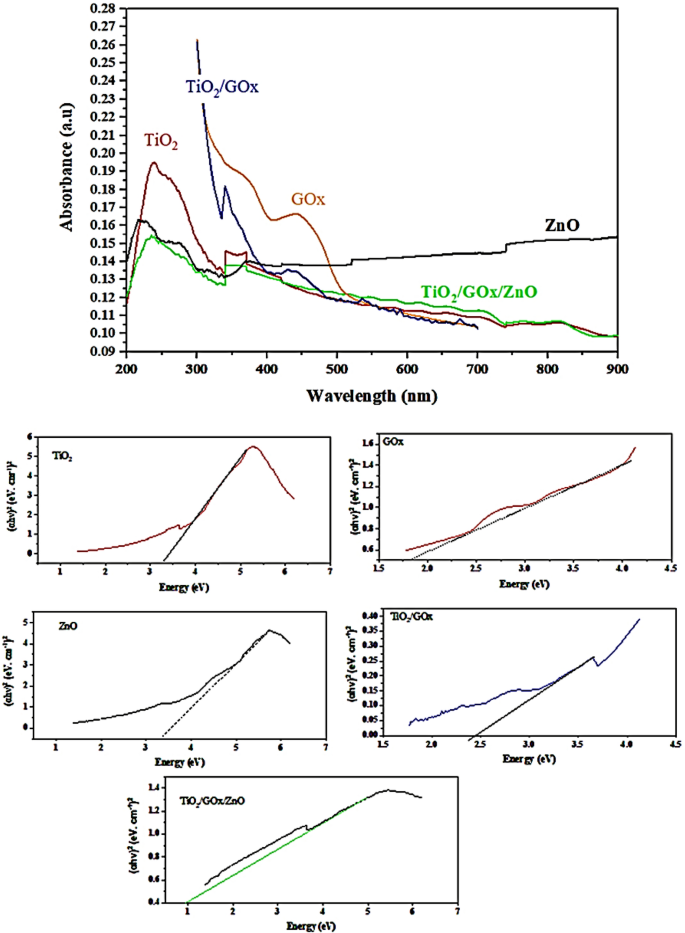
UV–Vis diffuse reflectance absorption spectra (DRS) of TiO2, GOx, ZnO, TiO2/GOx, TiO2/GOx/ZnO within the vary of 300–700 nm are proven in Fig. 10. Anatase TiO2, which is synthesized at 70 °C is utilized on this check. For TiO2/GOx, a shift of the absorption edge to the seen gentle area is witnessed. After 420.6 nm, the adsorption depth ascribed to TiO2/GOx/ZnO is greater than naked TiO2.
The Tauc methodology relies on the belief that the energy-dependent absorption coefficient α might be expressed by (αhν)1/2 or (αhν)2 = B (hν-Eg) the place h is the Planck fixed, ν is the photon’s frequency, Eg is the band hole vitality, and B is a continuing52.The corresponding band-gap vitality, calculated by utilizing the Kubelka–Munk (KM) methodology and the plot of (αhν)2 versus the photon vitality (hν) for pure TiO2 and ZnO are 3.20 and three.33 eV, respectively. ZnO/TiO2 heterojunction composite fibers has a bandgap of two.9 eV53. Binary composite of TiO2/GOx has a bandgap of two.43 eV, whereas for the TiO2/GOx/ZnO, bandgap is as little as 1.00 eV. Triple-heterojunction can enhance the migration of photo-excited cost carries amongst totally different elements to reinforce photo-activity and cost separation54.
Bio-photo-catalytic degradation of amoxicillin
Amoxicillin artificial wastewater with concentrations of 10–50 ppm is injected into the bio-photo-catalyst coated microchannel at a velocity of 0.5 mm/min. The multiphase response has been carried out for 1 h below UV gentle irradiation (0.25 W/cm3).
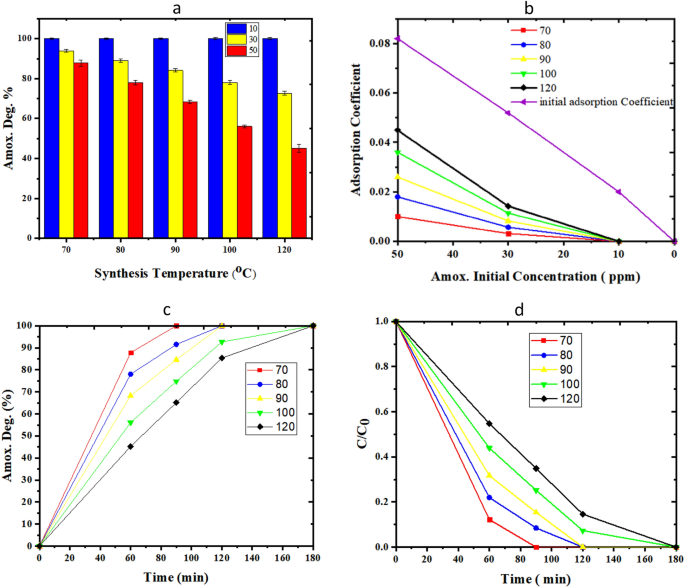
Determine 11a investigates the impact of synthesis temperature (70–120 °C) on the bio-photo-catalyst exercise. In the entire vary of synthesis temperature, can totally degrade amoxicillin as much as 10 ppm. Determine 11a makes clear that on the identical operational time, the decrease focus of amoxicillin and the decrease synthesis time of TiO2, the upper the decomposition yield. The extra pronounced antagonistic affect of increments in synthesis temperature rise might be noticed in circumstances with nice preliminary concentrations of amoxicillin. The impact of temperature might be interpreted by the SEM outcomes of photo-catalysts synthesized within the microfluidic system. The photo-catalysts that are synthesized at 70 °C have the smallest diameter and size and measurement distribution. Determine 11b offers extra particulars on the lower of amoxicillin adsorption coefficient at totally different preliminary amoxicillin concentrations. Determine 11c, d examine the impact of time on amoxicillin degradation over the bio-photo-catalysts with synthesis temperatures of TiO2 within the vary of (70–120 °C). In accordance with the pseudo first-order response, the plot of ln(C0/C) in opposition to time ought to be linear. Kinetics of response reveal that obvious fee constants for samples (70–120 °C) are as 0.035, 0.027, 0.0206, 0.0206, 0.0152 min−1, respectively. Within the meantime, the conduct of the pattern with 70 °C synthesis temperature defines with the best yield and most quickly degradation of amoxicillin.
Analysis of TiO2/ZnO/GOx bio-photo-catalyst assay in Amoxicillin degradation, (a, b) the impact of synthesis temperature and preliminary amoxicillin (10–50 ppm) degradation over 1 h, (c) the impact of operational time on amoxicillin degradation (0–180 min, preliminary focus of fifty ppm), (d) CAmox./CAmox., 0 verses time.
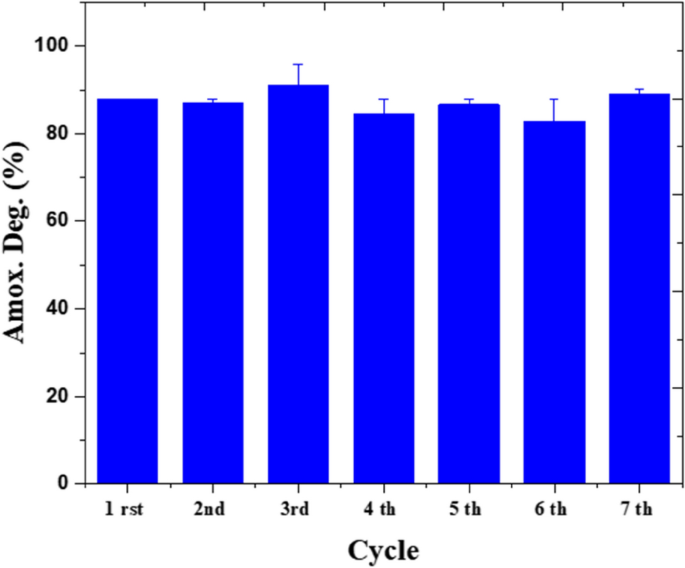
For stability check, amoxicillin resolution of fifty ppm illuminated by 0.25 W/cm3 is fed up into the microreactor at 0.5 mm/min. In these sequence of experiments, the operational stability of the system in seven consecutive cycles is studied. The residence time was 1 h and no rising has been performed between cycle intervals. On this work, solely 8.1% alteration is seen within the amoxicillin degradation effectivity (Fig. 12). Related outcomes have been noticed in the place tetracycline is to be degraded in a microfluidic programs over ZnO/ZnS by 5% change within the effectivity after 5 cycles55. Microfluidic reactors present uniform circumstances of sunshine propagation and movement distribution, which end in homogenous photoactivation of web sites, common interplay between energetic websites and pollution, and ultimately these programs permit greater stability in comparison with bulk reactors.
Desk 3 summarizes the revealed articles on the photo-catalytic amoxicillin degradation. In a latest work, using business titanium dioxide immobilized on the membrane to decompose amoxicillin (50 ppm) is reported through which, it’s eliminated as much as 80% after 500 min56. In one other work, a hybrid nanostructure of TiO2/WO3 with a calcination temperature of 700 °C has been utilized at a dose of 0.1 g/l to decompose 25 L of amoxicillin (100 ppm) and the depth of UV photo voltaic radiation was set to a continuing worth, on a pilot scale. They achieved an effectivity of 64%57. TiO2 as a slurry of titanium dioxide (anatase) at a response time and lightweight depth lower than the beforehand said articles, confirmed a 70% effectivity within the decomposition of amoxicillin, however its separation from the answer for reuse of photo-catalysts is harder58. The slurry pattern had a yield discount of about 13% below comparable working circumstances, besides that it was mounted on silica gel granules59. Publicity to seen gentle confirmed a big impact of cobalt promoter on rising the efficiency of TiO2 within the decomposition of amoxicillin60, which enhanced the effectivity by as a lot as 70% improve in comparison with TiO2. In one other work, bismuth (as a promoter) and platinum had been used to amplify TiO2 within the photo-catalytic evaluation of amoxicillin (10 ppm), which achieved an effectivity of 87% in 120 min below seen gentle61.
Within the current work, a TiO2- based mostly bio-photo-catalyst coated capillary has been attained by modification of TiO2 with glucose oxidase and oxide nanoparticles, and the entire decomposition of amoxicillin was irradiated with a 0.25 W/cm3 UV-light supply for 120 min at 50 ppm.
ZnO is dopant and it isn’t as influential as TiO2 is. Due to this fact, its impact on the outcome just isn’t that outstanding. Nonetheless, it’s price finding out. Two findings are concluded from Desk 4. First, it recommends that the microfluidic system is a greater setting for ZnO synthesis. Second, measurement distribution is the important thing property for attaining greater effectivity. In one other phrase, by tuning the movement charges, a desired measurement distribution and uniformity might be attained. As an illustration, as soon as the volumetric movement fee of NaOH is ready at 50 µl/min and ZnCl2 at 25 µl/min, a higher amoxicillin degradation and narrower measurement distribution are attained than the case through which each reagents collide with one another at 50 µl/min flowrate.