Impact of LXN deficiency on hematopoietic stem cells in bone marrow and immune cells in peripheral blood
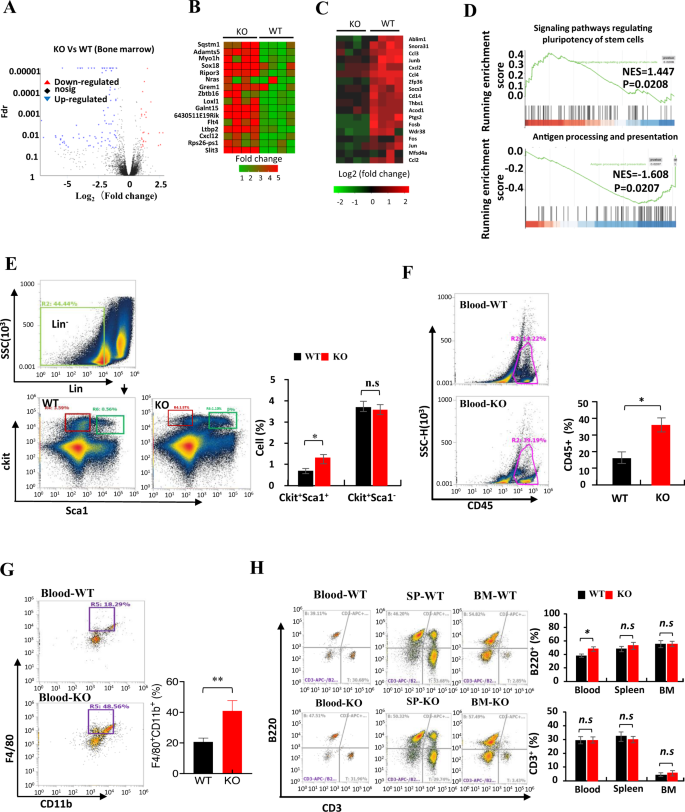
To start to grasp the impact of LXN knockout on immune system, we sequenced the transcriptome of WT and LXN−/− (KO) bone marrow (BM) by RNA-seq assay and analyzed the distribution of immune cells in several tissues by FACS assay. Our RNA-seq information confirmed that LXN deficiency induced a big upregulation of 25 genes expression, together with the genes Sqstm1, Sox18, Grem1 and Slit3, and downregulation of 86 genes, comparable to Socs3, Thbs1, Fos and Jun (Fig. 1A–C). GSEA evaluation confirmed that the genes in signaling pathways regulating stem cell pluripotent have been enriched in LXN−/−BM, whereas genes concerned in antigen processing and presentation have been decreased (Fig. 1D). As anticipated, FACS evaluation confirmed that the proportion of Lin−Sca1+ckit+ (LSK) cells in LXN poor BM elevated considerably in contrast with wild-type mice (Fig. 1E), which is according to the report of Liu et al. [32], indicating the chance of elevated self-renewal of LXN−/− hematopoietic stem cells (HSCs). In peripheral blood, we noticed that the proportion of CD45+ cells, F4/80+CD11b+ cells and B220+ cells elevated in LXN−/− mice, whereas the proportion of T cells didn’t change (Fig. 1F–H). There was no important change within the proportion of B220+ cells and CD3+ cells in WT- and LXN−/−-BM and spleen (Fig. 1F). Collectively, these information demonstrated that LXN deficiency not solely elevated hematopoietic stem cells, but additionally affected the distribution of immune cells within the peripheral system, a minimum of partly.
A Volcano plot of gene expression modifications of bone marrow from WT and LXN KO mice. B, C Heatmap of RNA-seq information displaying the up- (B) and downregulation (C) genes in LXN deficiency bone marrow. D GSEA of RNA-seq information from the bone marrow of LXN KO versus WT mice utilizing the signaling pathways regulating stem cell pluripotent, pathways in antigen processing and presentation gene set annotated within the KEGG. NES, normalized enrichment rating. E Consultant FACS plots and frequencies of Lin−Sca1+ckit+ in bone marrow from WT and LXN KO mice. F, G Consultant FACS plots and frequencies of CD45+ (F), F4/80+CD11b+ (G) cells in peripheral blood from WT and KO mice. H Consultant FACS plots and frequencies of B220+ and CD3+ cells in peripheral blood, spleen and bone marrow from WT and LXN KO mice. n = 6, *P < 0.05, **P < 0.01, n.s. no significance.
Mice lack of LXN promotes the expansion of most cancers cells within the subcutaneous tumor mannequin
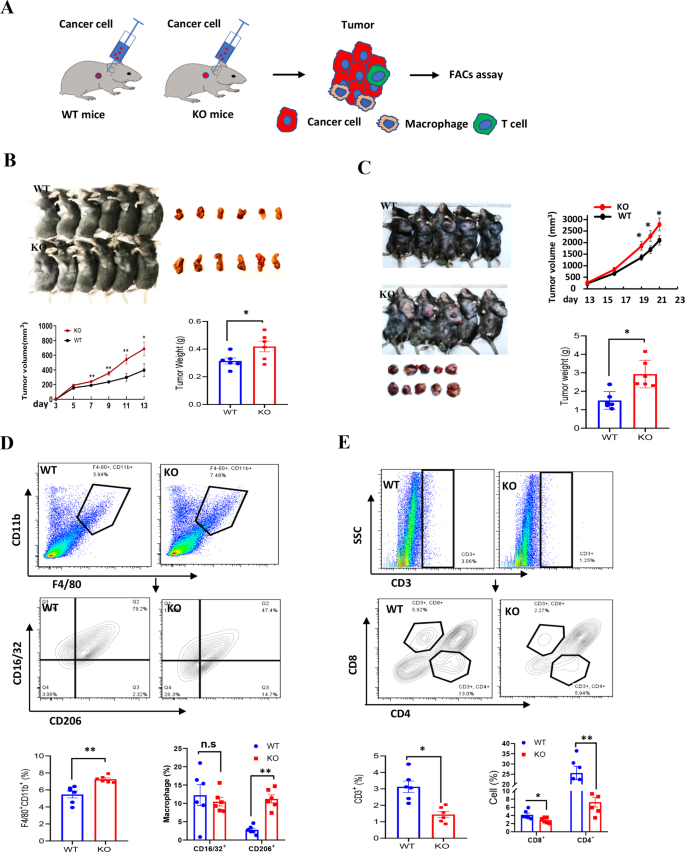
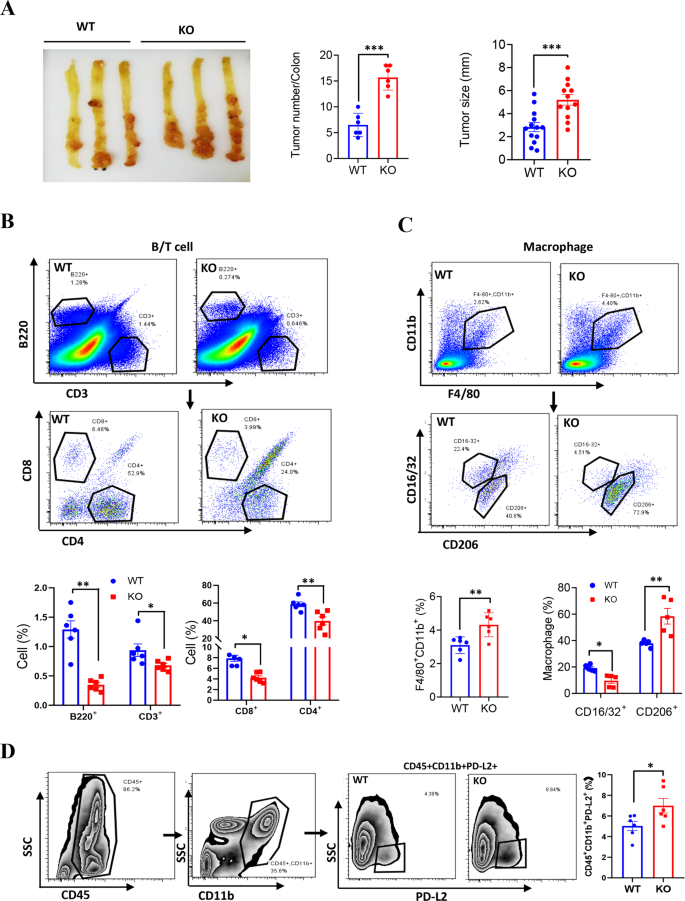
To guage the impact of LXN-deletion on mice immune system, we examined the most cancers cell progress in littermate wild-type (WT) and LXN−/− mice (KO) by subcutaneous tumor mannequin (Fig. 2A). Most cancers cells have been inoculated into the armpits of WT and LXN−/− mice. The outcomes confirmed that mice lack of LXN considerably promoted the expansion of most cancers cells over your entire assay interval in subcutaneous tumor mannequin, irrespective of loaded with colorectal most cancers or lung most cancers cells (Fig. 2B, C). FACs assay confirmed that the proportion of F4/80+CD11b+CD16/32−CD206+ macrophage (M2-macrophage) was remarkably elevated within the implanted tumors of LXN−/− mice (Fig. 2D), whereas T cell (CD3+CD4–CD8+ and CD3+CD8–CD4+) decreased considerably (Fig. 2E). Collectively, our information preliminarily demonstrated that LXN deficiency reworked the pro-tumor microenvironment of mice by selling the infiltration of M2-macrophages and attenuating T cells in tumor tissue, thus selling the expansion of subcutaneous tumor.
A Experimental scheme for subcutaneous tumor assay. B, C MC38 cells (B) and LLC cells (C) have been inoculated into the armpits of WT and LXN KO mice. The expansion of the implanted tumor dimension was measured each 3 days and the tumor weight was measured on the finish level (n = 6 mice). D Movement cytometry evaluation of macrophages (F4/80+CD11b+) and the totally different subsets (M1, CD16/32+ CD206−; M2, CD16/32−CD206+) in tumor from WT and LXN KO mice. E Movement cytometry evaluation of T cells (CD3+B220−) and the totally different subsets (CD8+T cell and CD4+T cell) in tumor from WT and LXN KO mice. Knowledge are consultant of three unbiased experiments. *P < 0.05, **P < 0.01, n.s. no significance.
LXN-deficient macrophage inhibits the operate of T cells in vitro
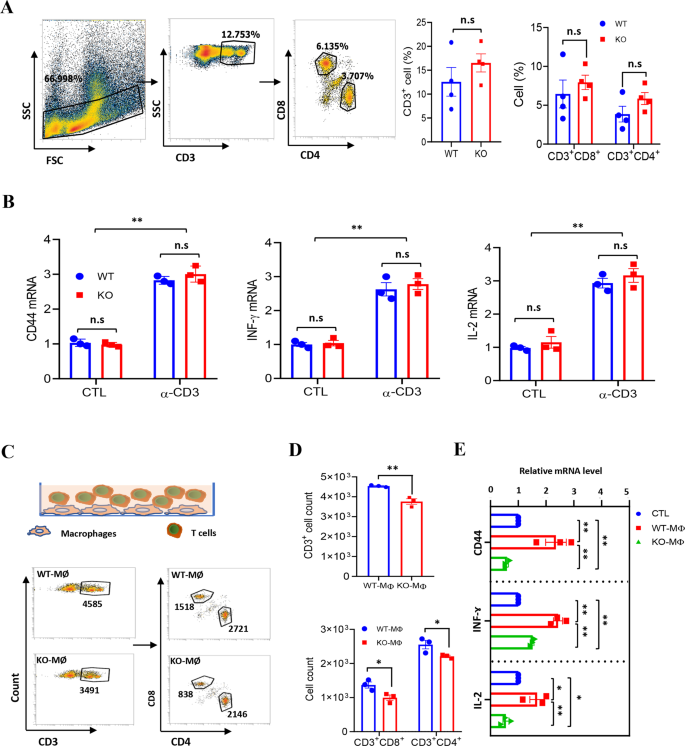
Because it was noticed that LXN deficiency would enhance macrophages and cut back the infiltration of T cells in subcutaneous tumors, we requested whether or not LXN deletion would change the operate of immune cells (comparable to macrophages and T cells) in vitro. We first evaluated the influence of LXN deficiency on T cells by analyzing the content material of T cells in spleen. We discovered that LXN knockout had no important impact on CD3+, CD3+CD8+ and CD3+CD4+ T cells in spleen (Fig. 3A). We additionally measured cytokines to characterize the exercise of WT and LXN−/− T cells. Our information confirmed that LXN knockout had no impact on T cell activation underneath basal or anti-CD3e antibody (α-CD3e) remedy (Fig. 3B). We then FACS-sorted BMDMs (F4/80+CD11b+) from WT and LXN−/− mice and labeled with CFSE. Subsequent, we co-cultured CFSE-labeled WT or LXN−/− BMDMs (WT-MØ or KO-MØ) with T cell (Fig. 3C). FACS assay present that CFSE–CD3+ cells, CFSE–CD3+CD4+ cells and CFSE−CD3+CD8+ cells decreased when co-cultured with KO-MØ (Fig. 3D). QPCR confirmed that the extent of CD44, INFɤ and IL-2 (Markers of T cell activation) have been a lot increased in WT-MØ-induced T cells than that of KO-MØ-induced (Fig. 3E), suggesting that T cells co-cultured with LXN−/− macrophages developed dysfunction, and existed in immunosuppressive state.
A Movement cytometry evaluation of T cells remoted from spleen of WT and LXN KO mice. Consultant FACS plots (left) and frequencies (proper) of T cells. n = 4, n.s. no significance. B T cells from WT and LXN KO mice have been activated by CD3 (5 μg/mL) antibodies for 48 h. The exercise of T cells was evaluated by measuring the expression of CD44, IFN-γ and IL-2, by qRT-PCR. n = 3, **P < 0.01, n.s. no significance. C–E Remoted T cells (1 ×;104 per properly) have been co-cultured in 96-well plates with a 2:1 ratio of the WT or LXN KO macrophage for 72 h. T cells have been analyzed by circulation cytometer (C), the rely of CD3+, CD3+CD4+ and CD3+CD8+ cells are confirmed (D). The manufacturing of CD44, INF-ɤ and IL-2 have been decided by qRT-PCR (E). n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, n.s. no significance. Knowledge are consultant of three unbiased experiments.
LXN deficiency enhances PD-L2 expression in macrophages and promotes M2 phenotype polarization
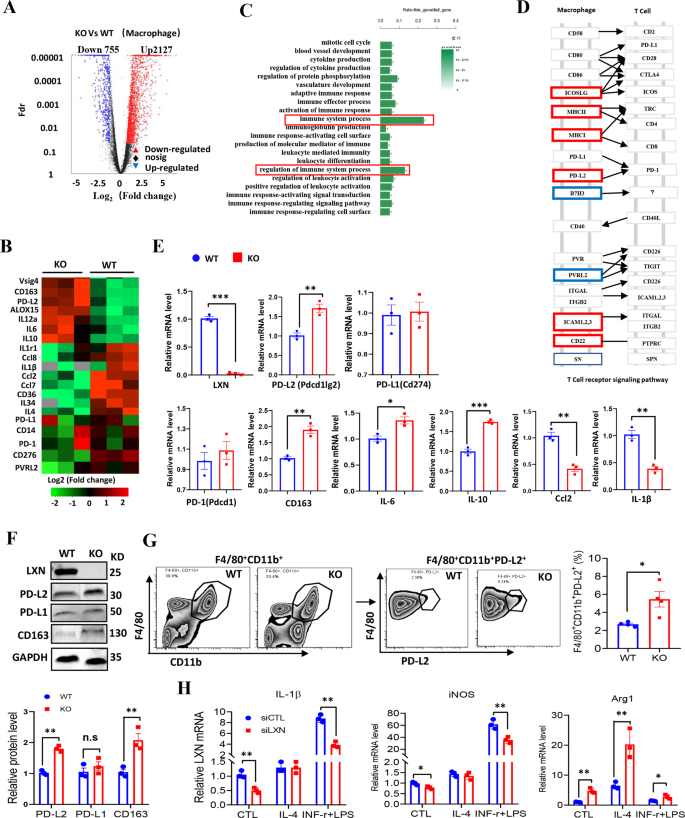
To grasp how LXN deficiency impacts macrophage operate, the gene profiling of bone marrow derived macrophages (BMDMs) from WT and LXN−/− mice was carried out by RNA-seq assays. Our information revealed that LXN deficiency induced a big upregulation of 2127 genes, together with Vsig4, ALOX15, PD-L2, CD163, and IL-10, and downregulation of 755 genes, comparable to CD276, IL-1β and Ccl2 (Fig. 4A, B). Gene ontology evaluation demonstrated that the genes concerned within the regulation of immune system course of have been most steadily regulated (Fig. 4C, D). The upregulation of PD-L2, CD163 and IL-10 and downregulation of Ccl2 and IL-1β in LXN deficiency BMDMs have been confirmed by qPCR (Fig. 4E). The expression of PD-L2 and CD163 in LXN−/− BMDMs have been additional confirmed by Western blot (Fig. 4F). The expression of PD-L2 in macrophage even be confirmed by FACs. We discovered that the F4/80+CD11b+ macrophage from the LXN−/− mice expressed increased degree of PD-L2 (F4/80+CD11b+PD-L2+) than that from WT mice (Fig. 4G).
A BMDMs from WT and LXN KO mice have been subjected to RNA-seq. Volcano plot of gene expression modifications in LXN KO and WT macrophages. B Heatmap of RNA-seq information displaying the up- and downregulation genes in LXN-deficient macrophages (n = 3 mice). C GO evaluation of upregulated and downregulated genes prompt the enrichment within the regulation of immune system. D Consultant KEGG evaluation of macrophage adhesion molecules in T cell receptor signaling pathway. E Relative expression ranges of cytokine and chemokine mRNA in WT and LXN KO macrophage have been measured by qRT-PCR. n = 3, *P < 0.05, **P < 0.01, ***P < 0.001. F Western blot confirmed LXN deficiency upregulates the expression PD-L2, PD-L1 and CD163 in macrophages. **P < 0.01, n.s. no significance. G Consultant FACS plots and frequencies of PD-L2+ populations in F4/80+CD11b+ cells from WT or LXN-deficient mice. n = 4, *P < 0.05. H Consultant qRT-PCR evaluation of M1 (IL-1β, iNOS) and M2 (Arg1) macrophage markers in RAW264.7 cells transfected with CTL or LXN siRNA after induction of IL-4 (20 ng/ml) or IFN-ɤ+LPS (30 ng/ml+100 ng/ml) for 72 h. n = 3, *P < 0.05, **P < 0.01. n = 3, **P < 0.01. Knowledge are consultant of three unbiased experiments.
Because the elevated expression of genes associated to M2-macrophage phenotype (comparable to Vsig4, ALOX15 and CD163) after LXN deletion, we additionally investigated whether or not LXN regulates macrophage polarization. RAW 264.7 cells have been stimulated with a mixture of IFN-ɤ and LPS to induce M1- or IL-4 to induce M2-macrophage polarization. We discovered that cells missing LXN confirmed a big enhance within the tendency to polarize in the direction of M2 phenotype, as monitored by Arg1 mRNA expression, however a lower within the tendency to polarize in the direction of M1 phenotype, as monitored by expression of iNOS and IL-1β mRNA (Fig. 4H). Collectively, our information demonstrated that LXN deficiency in macrophage promoted PD-L2 expression and elevated the tendency to polarize in the direction of M2 phenotype, suggesting LXN-deficient macrophages have a possible immunosuppressive phenotype.
LXN deficiency promotes PD-L2 expression relatively than PD-L1 by enhancing JAK1/STAT3 signaling pathway in macrophages
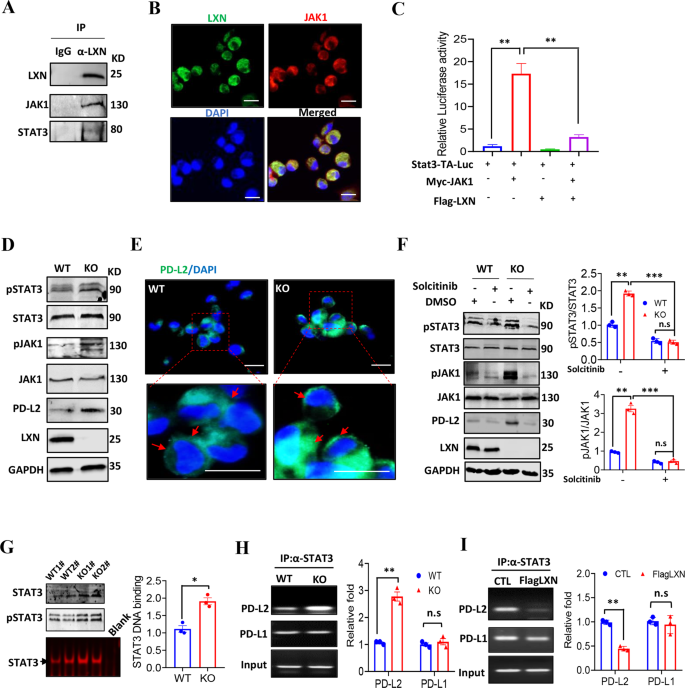
To raised perceive the molecule mechanism(s) underline LXN regulating macrophage, co-immunoprecipitation was carried out. We discovered that LXN, JAK1 and STAT3 kind a fancy in BMDMs (Fig. 5A). The interplay of LXN and JAK1 was additional confirmed in BMDMs by confocal (Fig. 5B). We carried out STAT3 binding sites-driven luciferase (Stat3-TA-Luc) assay in RAW264.7 cells, and located that overexpression of JAK1 considerably enhance the Stat3-TA-luc exercise, which was markedly inhibited when JAK1 and LXN have been co-expressed (Fig. 5C), indicating that LXN inhibited STAT3 transcriptional exercise by concentrating on JAK1. JAK/STAT has been reported to concerned within the regulation of PD-L1/L2 expression [33]. Our outcomes confirmed that LXN deletion in macrophage elevated expression of PD-L2, nonetheless, had no impact on the expression of PD-1 and PD-L1 (Fig. 4E, F). We speculated that this can be associated to the particular activation of STAT3 in LXN-deficient macrophages. We demonstrated that LXN deficiency in BMDMs considerably elevated the phosphorylation of JAK1 and STAT3, in addition to the expression of PD-L2 (Fig. 5D, E). As well as, the phosphorylation of JAK1 and STAT3 and the expression of PD-L2 brought on by LXN deficiency might be reversed by the remedy of macrophages with JAK1 inhibitor (Fig. 5F). These outcomes indicated that LXN was a suppressor of JAK1/STAT3 activation in macrophages. When LXN didn’t exist, the inhibitory impact of JAK1 was launched, thereby selling STAT3 exercise and PD-L2 expression. Due to this fact, our outcomes additional help the important thing function of LXN and JAK1 contribution to the exercise of STAT3 in macrophages.
A Immunoblotting exhibits that LXN interacts with JAK1 in BMDMs. B Consultant co-localization of LXN and JAK1 in BMDMs by immunofluorescence. Scale bars = 20 µm. C RAW264.7 cells have been transfected with pStat3-TA-luc, Myc-JAK1 and Flag-LXN plasmid as indicated, and induction of luciferase exercise was decided. Knowledge are consultant of three unbiased experiments. **P < 0.01. D Western blot exhibits that LXN deficiency will increase the expression of PD-L2 and phosphorylation of JAK1/STAT3 in mice macrophages. E Consultant the expression of PD-L2 in macrophages from WT and LXN KO mice by immunofluorescence. The purple arrow signifies that PD-L2 is expressed on the cell floor. Scale bars = 20 µm. F Macrophages from WT and LXN KO mice handled with solcitinib for twenty-four h, the expression of PD-L2 and the exercise of JAK1/STAT3 have been decided by Western blot (left). Quantitative evaluation of western blot was proven (proper). Knowledge are consultant of three unbiased experiments. **P < 0.01, ***P < 0.001, n.s. no significance. G Consultant the DNA-binding exercise of STAT3 in WT and LXN KO macrophage by EMSA assay. *P < 0.05. H Consultant the binding exercise of STAT3 to PD-L1/2 promoter in WT and LXN KO macrophages by ChIP assay. I Consultant the binding exercise of STAT3 to PD-L1/2 promoter in RAW 264.7 cells transfected with Flag LXN. Knowledge are consultant of three unbiased experiments. **P < 0.01, n.s. no significance.
STAT3 regulates many inflammatory- and immune-related genes [34, 35]. To additional characterize the transcriptional exercise of STAT3 in macrophage after LXN knockout, EMSA and ChIP assay have been carried out. The outcomes confirmed that LXN deficiency enhanced the DNA binding exercise of STAT3 in BMDMs (Fig. 5G), and intensely elevated the binding of STAT3 to PD-L2 promoter, however no considerably change of binding to the PD-L1 promoter (Fig. 5H). In distinction, overexpression of LXN attenuated the binding of STAT3 to PD-L2 promoter (Fig. 5I). Taken collectively, these findings collectively indicated that LXN interacted with JAK1 and inhibited the activation of JAK1/STAT3 signaling, thus negatively regulating the expression of PD-L2 in macrophages.
LXN deficiency remodels immune microenvironment by rising PD-L2+ macrophages and decreasing T cells infiltration in colorectal most cancers tissues in vivo
To find out whether or not PD-L2+ macrophages elevated in tumor microenvironment in LXN-deletion mice, we first generated AOM/DSS-induced colorectal most cancers mannequin in WT and LXN−/− mice (Supplementary Fig. S1A). We confirmed that LXN−/− mice developed extra extreme medical signs, together with fast weight reduction, a decrease survival fee, a better illness exercise index (DIA) and bloody stool, shortened colon and extra spleen achieve in contrast with WT mice (Supplementary Fig. S1B–G). Tumors occurred extra steadily, and tumor hundreds have been heavier in LXN−/− mice than in WT mice (Fig. 6A). These outcomes demonstrated that LXN-deficient mice have been extra prone to AOM/DSS-induced colorectal tumorigenesis in vivo. Then, major colorectal tumor tissues have been remoted from AOM/DSS-treated mice and analyzed by FACs assay. We discovered that B220+ cell and CD3+ T cell have been considerably diminished within the colorectal tumor of LXN−/− mice in comparison with WT mice (Fig. 6B). Nonetheless, F4/80+CD11b+ macrophages, significantly PD-L2+ macrophages (CD45+CD11b+PD-L2+) infiltrated within the colorectal tumor of LXN−/− mice was considerably increased than that in WT mice (Fig. 6C, D). Additional evaluation of subpopulations confirmed that F4/80+CD11b+CD16/32−CD206+ cell (M2-macrophage) was considerably elevated; nonetheless, F4/80+CD11b+CD206−CD16/32+ cell (M1-macrophage), CD3+CD4−CD8+ (CD8+T cell) and CD3+CD8–CD4+ (CD4+T cell) cells have been decreased in LXN−/− mice in comparison with that of WT mice (Fig. 6C, D). Collectively, these information implicated that LXN deficiency elevated PD-L2+ macrophages and decreased T cells infiltration in colorectal most cancers tissues, suggesting LXN deficiency enhances pro-tumor immune response in mice.
A Gross view of colonic tumor of AOM/DSS-treated WT and LXN KO mice. Tumor quantity and dimension have been counted (n = 6 mice). **P < 0.01. B Movement cytometry evaluation of B cell (CD3−B220+), T cells (CD3+B220−) and the totally different subsets (CD8+T cells and CD4+T cells) in colon tissue in WT and LXN KO mice handled with AOM/DSS. C, D Movement cytometry evaluation of macrophages (F4/80+CD11b+), the totally different subsets (M1, CD16/32+CD206−; M2, CD16/32−CD206+) (C) and CD45+CD11b+PD-L2+ cells (D) in colon tissue in WT and LXN KO mice handled with AOM/DSS. n = 6, *P < 0.05, **P < 0.01. Knowledge are consultant of three unbiased experiments.
LXN deficiency in hematopoietic lineage exacerbates tumorigenesis in vivo
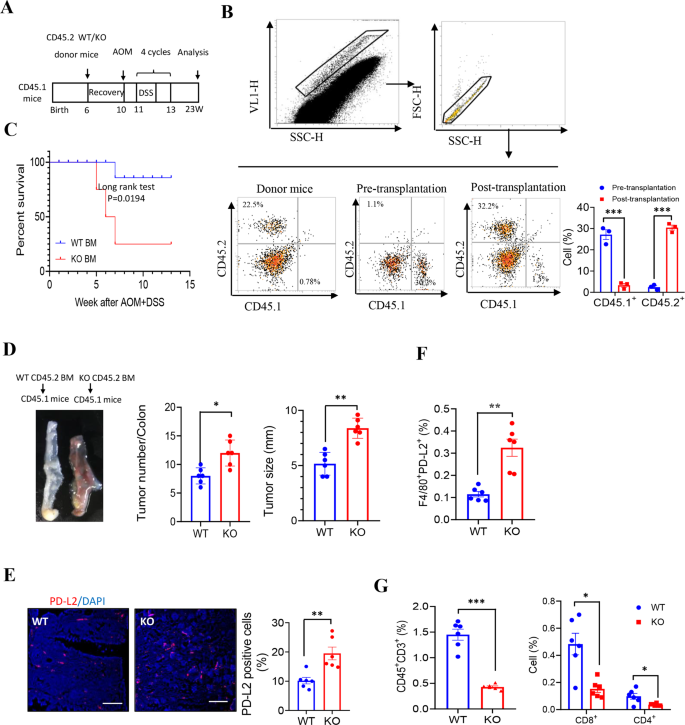
To verify the function of macrophage-derived LXN in tumor immunomodulation, bone marrow transplantation (BMT) was carried out. Donor BM from WT or LXN−/−mice (CD45.2 background) was engrafted into lethally irradiated host mice (CD45.1 background). After 4-week restoration, mice have been placed on 4 cycles AOM/DSS induction (Fig. 7A). Movement cytometric evaluation of CD45.2+ cells in blood to substantiate the success of transplantation (Fig. 7B). After AOM/DSS remedy, mice acquired LXN−/−BM exhibit considerably shortened survival (Fig. 7C). Constantly, the tumor hundreds and tumor dimension have been increased in mice acquired LXN−/−BM than in mice acquired WT BM (Fig. 7D). We additionally noticed a considerably elevated of PD-L2+ cells within the colorectal tumor tissues in AOM/DSS-induced mice acquired LXN−/−BM (Fig. 7E, F). As anticipated, T cell (CD45+CD3+), the subpopulation of CD8+ T cell (CD3+CD4−CD8+) and CD4+ T cell (CD3+CD8−CD4+) have been decreased in mice transplanted with LXN−/−BM (Fig. 7G). These outcomes of bone marrow chimeras strongly prompt that LXN-poor hematopoietic lineage accelerated AOM/DSS-induced colorectal tumorigenesis in mice.
A Schematic illustration of mouse mannequin of bone marrow transplantation. Bone marrow transfers have been carried out in 6-weeks outdated lethally irradiated WT mice (CD45.1 background) adopted by reconstitution with bone marrow from WT or LXN KO mice (CD45.2 background), respectively. Then, 4 cycles of AOM/DSS remedy to induce colorectal most cancers. B Consultant FACS plots present CD45.2+ and CD45.1+ cells within the peripheral blood of donor mice and recipient mice earlier than and after bone marrow transplantation, respectively. C Survival of AOM/DSS-treated mice (CD45.1 background) after performed transplantation with WT and LXN KO bone marrow (CD45.2 background) (n = 18 mice per group). D Consultant pictures of colorectal tissue of every group chimeras and tumor quantity and dimension have been counted. n = 6, **P < 0.01, ***P < 0.001. E Consultant pictures of cross-sections of the tumor tissues from every group stained with anti-PD-L2 antibody. Quantification of PD-L2 optimistic cells are proven. Scale bars = 100 µm. n = 6, **P < 0.01. F, G Frequencies of WT or LXN KO donor (CD45.2)-derived F4/80+PD-L2+ cells (F), CD3+, CD3+CD8+ and CD3+CD4+ T cells (G) within the tumor of recipient mice (CD45.1) after 4 cycles of AOM/DSS remedy. n = 6, *P < 0.05, **P < 0.01, ***P < 0.001. Knowledge are consultant of three unbiased experiments.
Adoptive remedy with WT macrophages rescues the operate of T cells in LXN-deficient mice
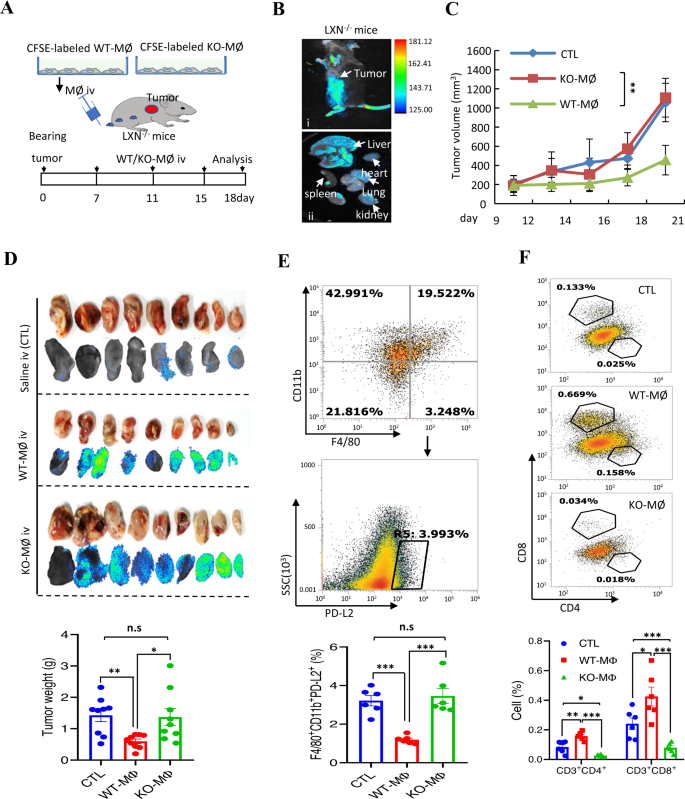
To additional show the inhibitory impact of LXN-deficient macrophages on tumor immunity, adoptive remedy with WT macrophages was carried out on tumor bearing LXN−/− mice. BMDMs from WT and LXN−/− mice have been labeled with CFSE. Most cancers cells have been engrafted into the subcutaneously of LXN−/− mice. After 7 days, the CFSE-labeled WT or KO-MØ have been injected by means of the tail vein on the indicated time factors (Fig. 8A). Animal imaging present that CFSE-labeled macrophages have been enriched in tumor websites (Fig. 8B-i), in addition to enriched within the liver (Fig. 8B-ii). After 18 days, giant variety of CFSE-labeled macrophages have been noticed in tumor tissue, and the tumors of WT-MØ-treated group have been considerably smaller than that of KO-MØ-treated group (Fig. 8C, D), indicating that KO-MØ possessed the traits of tumor related macrophages (TAM). In contrast with the KO-MØ injection group, fewer F4/80+CD11b+PD-L2+ macrophages might be detected within the WT-MØ injection group (Fig. 8E). Upon analyzing T cell infiltration in tumor tissue, we discovered that, as anticipated, WT-MØ injected into LXN−/− mice elevated CD3+CD4+ and CD3+CD8+ T cells in tumor tissue, nonetheless, KO-MØ additional attenuated T cells (Fig. 8F), supporting the speculation that LXN-deficient macrophages inhibited T cells, whereas WT-MØ might rescue the operate of T cells in LXN−/− mice, a minimum of in partial, in tumor microenvironment.
A Schematic illustration of the mouse mannequin of cell adoptive remedy for within the subcutaneous tumor fashions. Macrophages remoted from bone marrow of WT and LXN KO mice have been labeled with CFSE. MC38 cells have been engrafted into the subcutaneously of LXN KO mice. The CFSE-labeled WT or LXN KO-macrophages have been injected twice by means of the tail vein on the indicated time factors. B Animal imaging exhibits the enrichment of CFSE-labeled macrophages on the tumor web site (i) and different organs (ii), as proven by the white arrow. C Consultant the tumor progress curves. n = 9, *P < 0.05, **P < 0.01, n.s. no significance. D Imaging of tumor tissues of tumor-bearing recipient LXN KO mice that have been handled with CFSE-labeled WT or LXN KO-Macrophages, and the tumor weight on the finish level was proven. n = 9. E Consultant FACS plots and frequencies of F4/80+CD11b+PD-L2+ macrophages in subcutaneous tumors handled with WT or LXN KO-Macrophages. n = 6, ***P < 0.001, n.s. no significance. M Consultant FACS plots and frequencies of CD3+CD4+ and CD3+CD8+ T cells in subcutaneous tumors handled with WT or LXN KO-Macrophages. n = 6, *P < 0.05, **P < 0.01, ***P < 0.001.
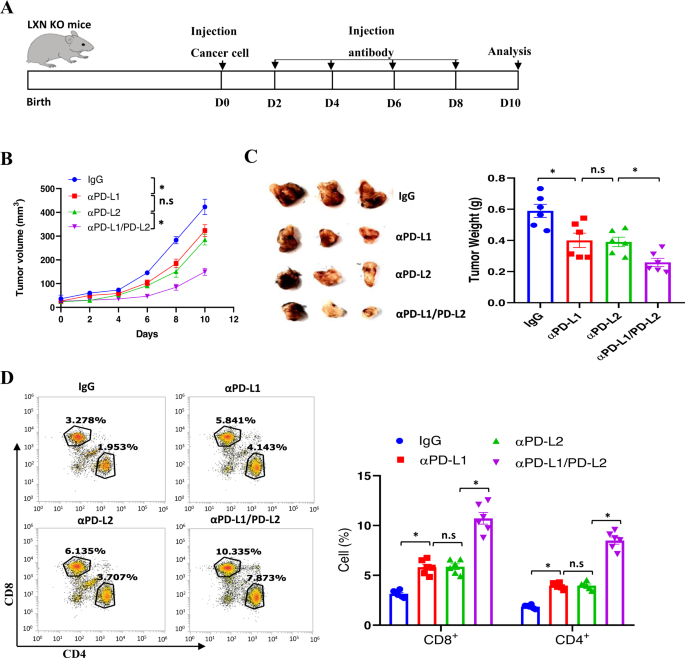
PD-L2 blockading attenuates colorectal most cancers cell progress in LXN-deficient mice
The PD-L1/2/PD-1 axis represents an immune-inhibiting checkpoint mediating tumor immune evasion [18, 33]. We noticed the elevated PD-L2 ranges in LXN-deficient macrophage, we accordingly evaluated the consequences of PD-L2 and PD-L1 blockade on most cancers progress. To this finish, MC38 cells have been engrafted into the subcutaneously of LXN−/− mice. After 2 days, these mice have been injected intraperitoneally with a 20 mg/kg dose of monoclonal anti-PD-L1, anti-PD-L2, or anti-PD-L1/PD-L2 blocking antibody, IgG as management antibodies, each 2 days, for a interval of 10 days, then tumor tissues have been collected and decided (Fig. 9A). Anti-PD-L2-treated mice displayed important discount within the progress of tumor quantity over your entire assay interval and tumor weight on the finish level in subcutaneous fashions in contrast with management antibody-treated mice, though there was no important distinction between the anti-PD-L1 and anti-PD-L2 handled teams (Fig. 9B). Notably, we noticed that the mix of anti-PD-L1 and anti-PD-L2 has the perfect tumor therapeutic impact (Fig. 9B, C). In line with these outcomes, our information confirmed that CD8+ and CD4+ T cells have been considerably increased in anti-PD-L2 or anti-PD-L1/PD-L2 handled group in contrast with management group (Fig. 9D). Taken collectively, our information from antibody remedy additional demonstrated that the rise of PD-L2 in LXN-deficient macrophage was vital for tumorigenesis.
A Experimental time line for the implantation of MC38 cells in LXN KO mice and therapeutic schedule. B Most cancers cell bearing mice have been handled with anti-PD-L1, anti-PD-L2, anti-PD-L2/PD-L2, or IgG management. The expansion of the implanted tumor dimension was measured each 2 days. n = 6, *P < 0.05, n.s. no significance. C Consultant pictures of mice bearing tumor shaped by MC38 cells, and quantification of tumor weight on the finish level after antibody remedy. n = 6, *P < 0.05, n.s. no significance. D Consultant dot plots and of quantification of CD4+ and CD8+ T cells in tumor after antibody remedy. n = 6, *P < 0.05, n.s. no significance.