In Down syndrome, the third copy of chromosome 21 causes a reorganization of the 3D configuration of the complete genome in a key cell sort of the growing mind, a brand new research exhibits. The ensuing disruption of gene transcription and cell perform are so much like these seen in mobile getting old, or senescence, that the scientists main the research discovered they may use anti-senescence medication to right them in cell cultures.
The research printed in Cell Stem Cell subsequently establishes senescence as a doubtlessly targetable mechanism for future therapy of Down syndrome, says Hiruy Meharena, who led the work as a Senior Alana Fellow within the Alana Down Syndrome Middle at MIT and is now an assistant professor on the College of California at San Diego.
“There’s a cell-type-specific genome-wide disruption that’s unbiased of the gene dosage response,” Meharena says. “It’s a really related phenomenon to what’s noticed in senescence. This means that extreme senescence within the growing mind induced by the third copy of chromosome 21 may very well be a key cause for the neurodevelopmental abnormalities seen in Down syndrome.”
The research’s discovering that neural progenitor cells (NPCs), which grow to be main cells within the mind together with neurons, have a senescent character is exceptional and novel, says senior creator Li-Huei Tsai, however it’s substantiated by the crew’s in depth work to elucidate the underlying mechanism of the results of irregular chromosome quantity, or aneupoloidy, inside the nucleus of the cells.
“This research illustrates the significance of asking basic questions concerning the underlying mechanisms of neurological issues,” says Tsai, Picower Professor of Neuroscience, director of the Alana Middle, and of the Picower Institute for Studying and Reminiscence at MIT. “We didn’t start this work anticipating to see senescence as a translationally related function of Down syndrome, however the information emerged from asking how the presence of an additional chromosome impacts the structure of all of a cell’s chromosomes throughout improvement.”
Genome-wide adjustments
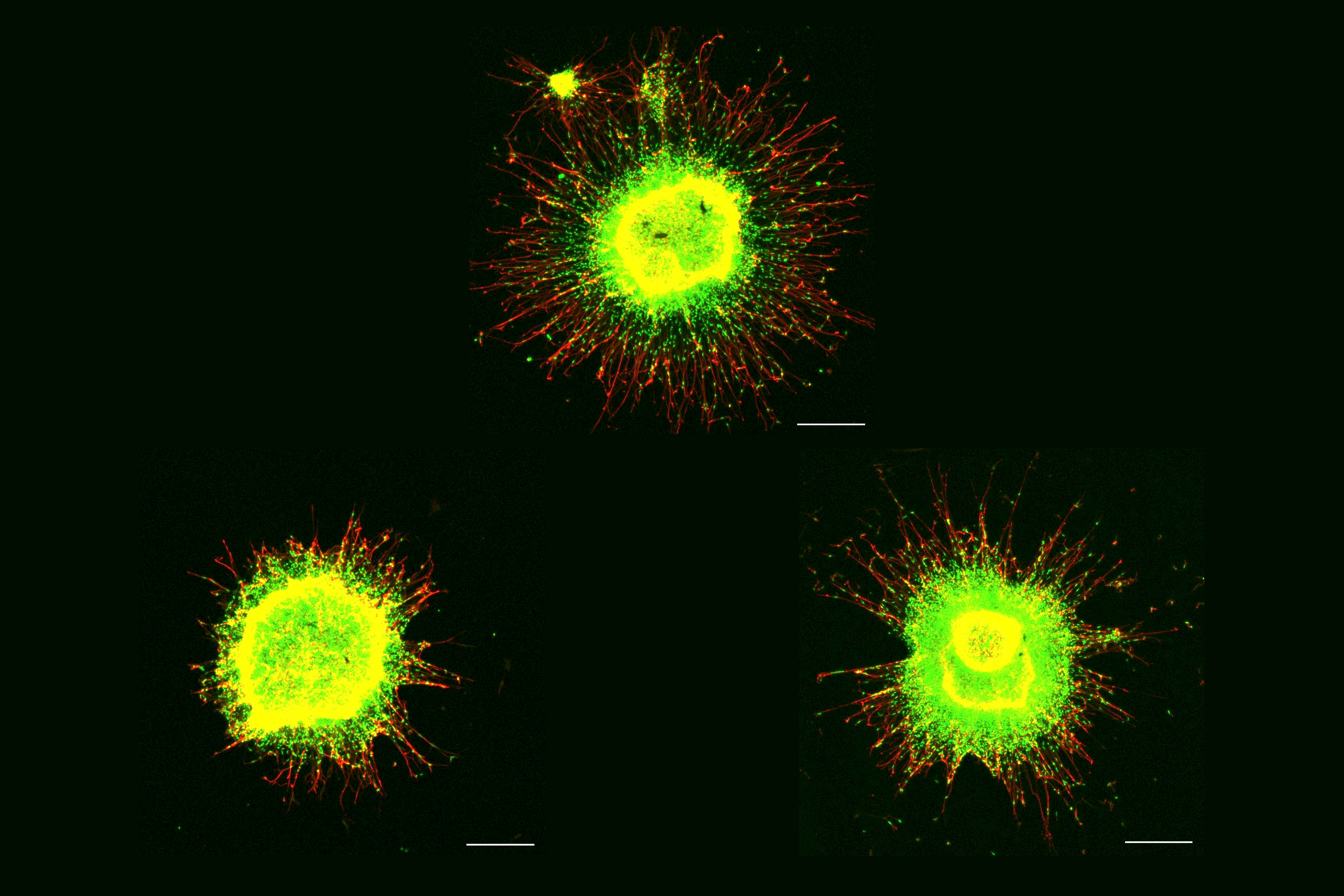
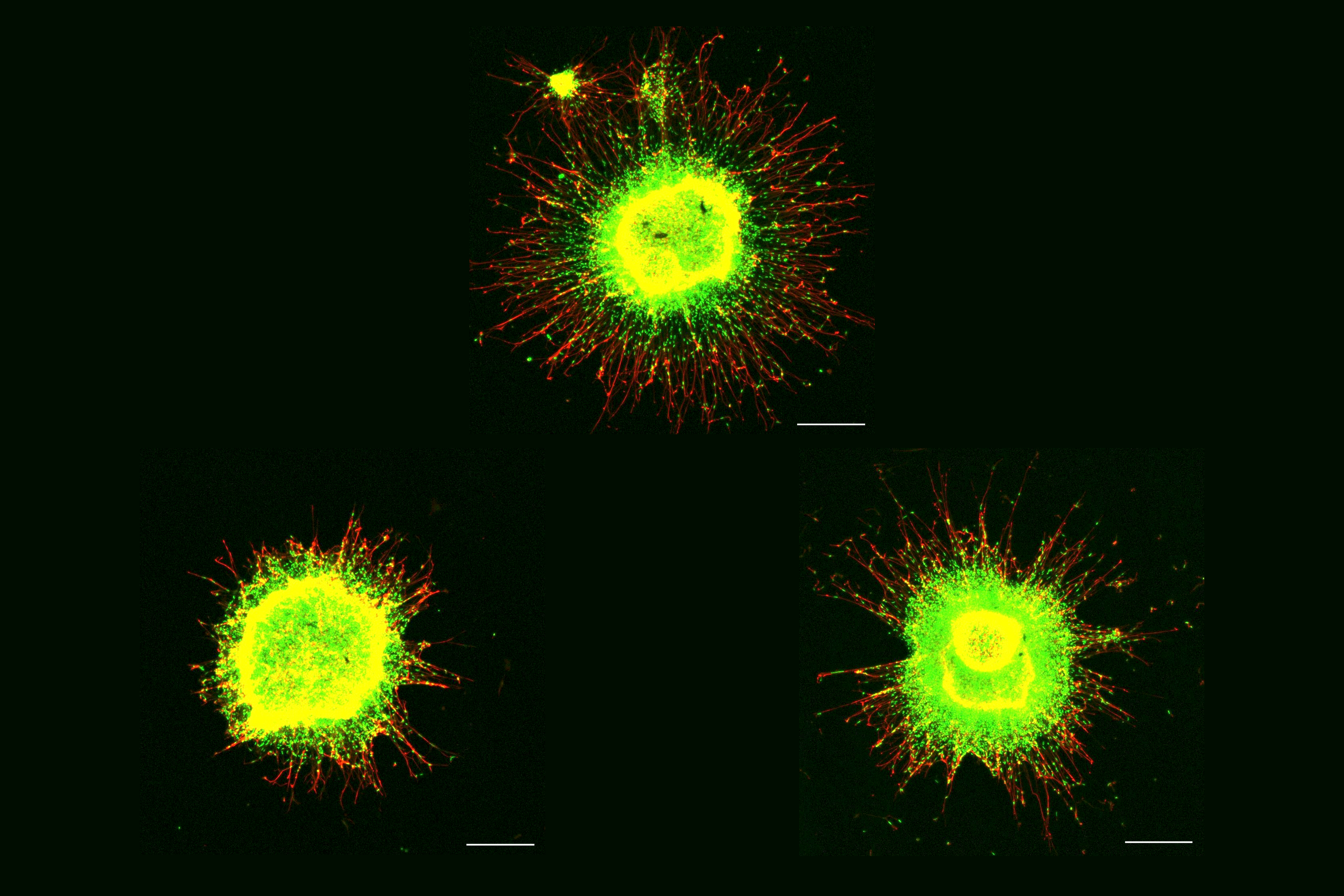
Meharena and co-authors spent years measuring distinctions between human cell cultures that differed solely by whether or not they had a 3rd copy of chromosome 21. Stem cells derived from volunteers have been cultured to show into NPCs. In each the stem cells and the NPCs, the crew examined 3D chromosome structure, a number of metrics of DNA construction and interplay, gene accessibility and transcription, and gene expression. In addition they appeared on the penalties of the gene expression variations on essential capabilities of those developmental cells, resembling how properly they proliferated and migrated in 3D mind tissue cultures. Stem cells weren’t notably completely different, however NPCs have been considerably affected by the third copy of chromosome 21.
Total, the image that emerged in NPCs was that the presence of a 3rd copy causes all the opposite chromosomes to squish inward, not in contrast to when folks in a crowded elevator should slender their stance when yet one more individual squeezes in. The primary results of this “chromosomal introversion,” meticulously quantified within the research, are extra genetic interactions inside every chromosome and fewer interactions amongst them. These adjustments and variations in DNA conformation inside the cell nucleus result in adjustments in how genes are transcribed and subsequently expressed, inflicting essential variations in cell perform that have an effect on mind improvement.
Handled as senescence
For the primary couple of years as these information emerged, Meharena says, the total significance of the genomic adjustments weren’t obvious, however then he learn a paper displaying very related genomic rearrangement and transcriptional alterations in senescent cells.
After validating that the Down syndrome cells certainly bore such an analogous signature of transcriptional variations, the crew determined to check whether or not anti-senecence medication might undo the results. They examined a mixture of two: dasatinib and quercetin. The medicines improved not solely gene accessibility and transcription, but in addition the migration and proliferation of cells.
That stated, the medication have very important uncomfortable side effects — dasatinib is barely given to most cancers sufferers when different remedies haven’t executed sufficient — so they don’t seem to be acceptable for making an attempt to intervene in mind improvement amid Down syndrome, Meharena says. As a substitute, an end result of the research may very well be to encourage a seek for medicines that would have anti-senolytic results with a safer profile.
Senescence is a stress response of cells. On the similar time, years of analysis by the late MIT professor of biology Angelika Amon, who co-directed the Alana Middle with Tsai, has proven that aneuploidy is a supply of appreciable stress for cells. A query raised by the brand new findings, subsequently, is whether or not the senescence-like character of Down syndrome NPCs is certainly the results of an aneuploidy-induced stress and, if that’s the case, precisely what that stress is.
One other implication of the findings is how extreme senescence amongst mind cells would possibly have an effect on folks with Down syndrome later in life. The chance of Alzheimer’s illness is far larger at a considerably earlier age within the Down syndrome inhabitants than amongst folks on the whole. Largely that is believed to be as a result of a key Alzheimer’s danger gene, APP, is on chromosome 21, however the newly recognized inclination for senescence might also speed up Alzheimer’s improvement.
Along with Meharena and Tsai, the paper’s different authors are Asaf Marco, Vishnu Dileep, Elana Lockshin, Grace Akatsu, James Mullahoo, Ashley Watson, Tak Ko, Lindsey Guerin, Fatema Abdurrob, Shruti Rengarajan, Malvina Papanastasiou and Jacob Jaffe.
The Alana Basis, the LuMind Basis, Burroughs Wellcome Fund, UNCF-Merck, and the Nationwide Institutes of Well being funded the analysis.