Bored with your experiments failing for no obvious cause? Options stopped working after only a few weeks?
Appears like you may have a buffer downside!
Whether or not you’re pulling your hair out and suspect your buffer is the difficulty, or whether or not you simply want to have the ability to competently reply “how do buffers work?” earlier than your subsequent assembly—we’ve acquired your again.
Right here’s a brief sharp lesson on buffers, how they work, and choose the suitable one for you.
What Is a Buffer?
A buffer is an answer containing acid and a proportionate quantity of conjugate base able to sustaining a secure pH when a small quantity of further acid or base is added to it.
It’s a easy definition, however the underlying chemistry is just a little bit difficult. We’ll clarify it shortly.
Acids and Bases: A Refresher
Keep in mind that acids donate protons, and bases settle for protons.
The stronger the acid, the extra readily it should dissociate into protons and anions. Stronger bases usually tend to dissociate into cations and proton acceptors.
The energy of an acid or base is measured by its dissociation fixed, or pKa. The decrease the pKa, the extra acidic the molecule.
How Do Buffers Work?
Let’s illustrate this through the use of the dissociation of water for example. We will categorical the dissociation of water by writing the next equation:
H2O –> OH– + H+
Water dissociates into hydroxy anions (OH–) and protons (H+).
OH– is a powerful base, and H+ is a powerful acid (the strongest, actually, being a proton).
A small buffering motion happens right here as a result of water can dissociate into a powerful acid and a powerful base, however stability one another out completely (as a result of each of those merchandise got here from the identical mother or father water molecule).
In different phrases, the water to the left provides extra OH- to the suitable to match the quantity of strongly acid protons, sustaining a continuing pH. In impact, self-buffering.
In sensible phrases, nevertheless, water is a particularly poor buffer. However we are able to obtain the buffering impact utilizing a number of completely different chemical compounds. You should have heard of the frequent examples utilized in your lab. Tris, acetic acid, citric acid, sodium phosphate, and HEPES, are among the extra frequent ones.
So, a buffer doesn’t maintain a given response solidly at a given pH however prevents wild swings within the acid-base stability.
Why Are Buffers Necessary to Your Experiments?
A buffer supplies a secure chemical surroundings on your experiments and reactions to happen in!
As soon as once more, let’s use an instance as an instance the significance of buffers and elaborate on the above level.
Suppose you might be culturing micro organism in a wealthy media that may assist the expansion of a number of cells. The cells will secrete numerous chemical compounds as they develop, stay, and die. That is simply a part of their metabolism and life cycle.
Lots of cells will secrete a number of chemical compounds.
A few of these chemical compounds will likely be acidic, and a few of these chemical compounds will likely be primary. Due to this fact, they’ll change the pH of the expansion medium—except there’s a buffer current to take care of it.
In any other case, the pH change will likely be detrimental to cell development sooner or later. That’s to say, the cells will die. Not good.
Bonus level for the observant readers—wealthy cell development media comparable to terrific broth are buffered utilizing sodium phosphate!
It’s not simply development media, although. Cellular phases in analytical chemistry, gel loading, enzyme assay, and protein crystallization options all comprise appropriate buffers to take care of their pH.
Buffers and Answer Longevity
There are passive results that buffers mitigate too. For instance, chemical compounds in your options will degrade over time, producing merchandise that alter their pH.
And atmospheric CO2 dissolves in water to generate carbonic acid, making your options extra acidic over time.
And there’s all the time that pesky floaty life that begins to develop in previous bottles; they will even change the pH of the answer.
Buffers and Species Protonation
Lots of chemical compounds we’re interested by as biologists have completely different protonation states. And people protonation states might be important for our experimental design and outcomes.
Take lysine, for instance. It has three protons that it will probably donate:
- The carboxyl proton.
- The ammonium proton.
- The aspect chain proton (additionally ammonium for lysine).
The carboxyl group is acidic by definition, and an answer with a pH > ~2.2 will deprotonate it.
The aspect chain ammonium group is primary, nevertheless. To deprotonate it, we’d like an answer with a pH > ~10.5. I.e., a reasonably primary answer.
Relying on our experimental goals, we’d need our lysine analyte to be absolutely protonated, deprotonated, or someplace in between.
We will obtain the specified protonation state through the use of a buffer.
Lysine is a crude instance, however SDS-PAGE and ion-exchange chromatography are two strategies by which management over species’ protonation is essential to success.
Try Determine 1 beneath to see the identical instance illustrated with the conjugate acids of buffers sodium phosphate and sodium citrate.
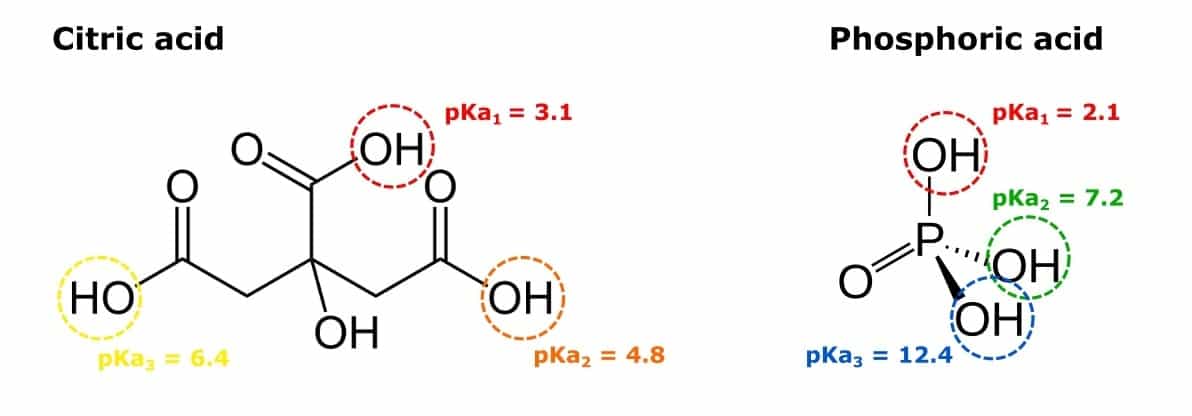
Each acids on this determine are triprotic and might donate three protons. With each proton that’s eliminated, the remaining protons are certain extra tightly to the acid molecule. To take away the third proton from phosphoric acid, a chemical with a pKa > 12.4 (a base) could be required.
Mixing mono, di, and tribasic types of these chemical compounds collectively in particular ratios permits us to tailor the pH of the buffer answer.
Buffers in Biology and Organic Buffers
Organic methods have peak exercise in a really pH slender vary (at a pH of about 7). That is just because most life on earth is water-based, and water has a pH of seven.
For instance, egg whites and seawater have a pH of ~ 8. Blood, sweat, and tears are nearer to 7.3. And milk is about 7. Gastric juices, nevertheless, are heavy in HCl and have a pH of just under 2.0.
So it’s no coincidence that the majority organic acids and bases are weak. However even a pH that’s solely barely decrease or greater than seven can influence your experiments.
Keep in mind that pH and buffer energy are calculated on a logarithmic scale. So selecting a buffer of applicable acidity/basicity is important.
The right way to Select an Applicable Buffer
So, you’re satisfied. Now you’re going to buffer all the pieces. However how do you choose an applicable buffer? And when you’ve chosen a buffer, how a lot do you have to add to your experiment?
Oh, and by applicable, I imply a buffer that may keep a pH vary that roughly matches your experiment’s supposed pH.
As an apart, did I point out that buffers have pH ranges—effectively, they do! Some have broad ranges and might function over a number of pH models. Some have slender ranges. You’ll know once you’ve exceeded the vary of a given buffer as a result of including additional acid or base will drastically change the pH (the other of what a buffer ought to do).
Anyway, to infer these two issues, you might want to know the damaging log of your buffer’s dissociation fixed, pKa. Often, you’ll be able to look this up on the web. Often, you haven’t any different alternative however to look it up, actually. However you’ll be able to calculate it utilizing the next equation if want be:
Ka = [H+][A–]/[HA].
Merely take the damaging logarithm of your Ka to get pKa.
To select an applicable buffer, choose one with a pKa that carefully matches the specified pH of your experiment. Easy.
And to calculate how a lot buffer so as to add to your experiment or how a lot acid or base so as to add to your buffer to realize the specified pH—I’ll maintain issues actually easy.
You may get all fancy and rearrange the equation above for the specified time period [H+], [A–], [HA]. Then you definately may go to the lab and begin faffing round including minute quantities of powder and liquid to your experiment.
Or you might:
- Dissolve a excessive focus of your chosen buffer in water.
- Titrate in conjugate acid or base till you obtain your required pH.
- Autoclave and retailer it.
- Add this inventory answer into your experiments to a wise focus.
Easy, eh!
Concerns for Selecting an Applicable Buffer
We simply defined the nitty gritty of selecting an applicable buffer, however there are different components to think about. Let’s group these with what we’ve discovered already:
- Select a buffer with a pKa that matches the pH of your supposed experiment.
- Select a buffer that doesn’t react together with your analytes.
- Select a buffer that’s appropriate together with your instrumentation.
- Beware that altering the temperature of the buffer adjustments its pH.
- Beware that neutralizing HCl with NaOH produces additional NaCl.
And that will help you a bit additional, right here’s a choice of Good’s buffers and their corresponding pKa. [1]
Desk 1. A non-exhaustive listing of Good’s buffers.
There’s Extra to Find out about Buffers
And that’s the reply to “how do buffers work?” together with a load of additional info that you just’ll hopefully discover helpful within the lab.
It doesn’t finish there, although! Why not learn to design the proper buffer answer on your experiments or examine what separates good buffers from excellent ones?
Bear in mind—utilizing the suitable buffer will result in extra profitable experiments sooner or later.
And remember to depart any questions within the feedback part beneath!
Initially revealed April 2012. Reviewed and up to date September 2022.
References
- Good NE et al. (1966) Hydrogen ion buffers for organic analysis. Biochem 5(2):467–77

