The actinomycete DEM30355 was remoted from a soil pattern, collected from the El Tatio geyser subject inside an arid a part of the Atacama Desert in Chile17. Pressure DEM30355 was recovered within the genus Amycolatopsis, primarily based on 16S rRNA evaluation, forming a subgroup with Amycolatopsis vancoresmycina DSM 44592 T and Amycolatopsis bullii SF27T (see ESI). The genus Amycolatopsis incorporates 94 species and 4 subspecies encompassing each extremophiles and producers of bioactive secondary metabolites, together with the clinically used vancomycin and rifamycin antibiotics19,20. Preliminary bioactivity screening confirmed that extracts of Amycolatopsis sp. DEM30355 displayed promising antibiotic exercise towards B. subtilis, thus we determined to look at the genome of Amycolatopsis sp. DEM30355 for novel biosynthetic potential. Purified genomic DNA from Amycolatopsis sp. DEM30355 was analysed utilizing each PacBio® and Illumina® sequencing applied sciences and genome meeting was carried out utilizing the mixed datasets to offer a 9.6 Mb draft genome, in 13 contigs. The draft genome of Amycolatopsis sp. DEM30355 was examined utilizing the secondary metabolite evaluation software program AntiSMASH 6.0.121. Of the 31 biosynthetic gene clusters (BGCs) detected, a PKS cluster was recognized exhibiting reasonable general similarity (81%) to that which encodes for rishirilides A and B22,23,24,25,26. These compounds are anthracenone polyketides, initially remoted from Streptomyces rishiriensis OFR-1056, with no reported antibiotic exercise. Rishirilide B has been proven to be a reasonably potent inhibitor of α2-macroglobulin, glutathione S-transferase and asparaginyl-tRNA synthetase, while little is thought concerning the organic position of rishirilide A (Fig. 1)18,27,28.
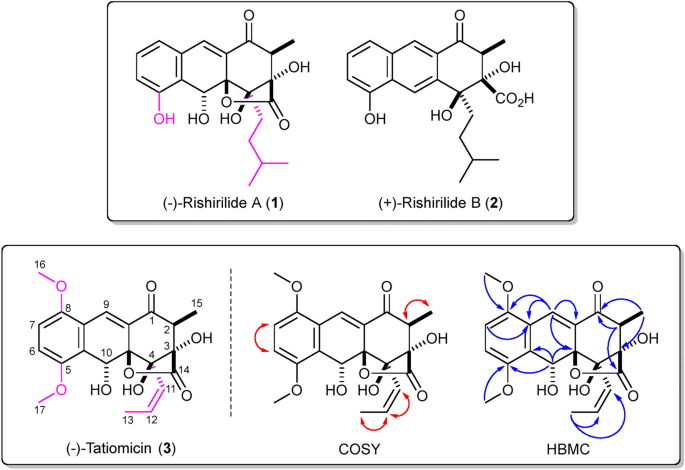
Prime (–)-Rishirilide A (1) and ( +)-rishirilide B (2). Relative stereochemistry of (–)-1 and absolute stereochemistry of ( +)-2 proven. Backside. Construction of (–)-tatiomicin (3) as derived from NMR and SCXRD experiments. Key COSY (crimson) and HMBC (blue) correlations proven. Absolute stereochemistry as proven by each vibrational round dichroism (VCD) and single-crystal X-ray diffraction (SCXRD) resonant scattering experiments. Structural variations of rishirilide A and tatiomicin are highlighted (magenta).
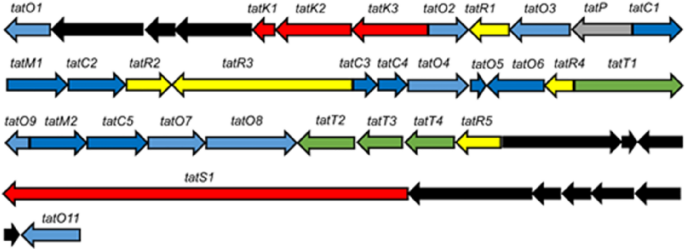
Additional inspection of the BGC from Amycolatopsis sp. DEM30355 revealed a extremely altered gene synteny (see ESI), in comparison with the rishirilide BGC, together with the presence of a number of new genes: one postulated to be concerned in PKS biosynthesis (tatS1), two encoding methyltransferases (tatM1 and tatM2), two encoding cyclases (tatC4 and tatC5) and one cytochrome p450 oxidoreductase (tatO11) (Fig. 2). As a result of important variation within the genetic make-up of the BGC, we postulated that it could code for the manufacturing of an as but undiscovered polyketide and as such we set about trying to establish this molecule from the metabolome of Amycolatopsis sp. DEM30355.
Group of the tatiomicin BGC. Genes coding for polyketide biosynthesis (crimson; tatS = starter unit biosynthesis, tatK = chain biosynthesis), polyketide modification (blue; tatO = oxidoreductases, tatC = cyclases, tatM = methyltransferases), regulation (yellow; tatR), transport (inexperienced, tatT) and others (gray; tatP = phosphorylase, black; genes not assigned to the tatiomicin BGC primarily based on homology to the rishirilide BGC and proposed biosynthetic pathway)).
Preliminary evaluation of the fermentation supernatant of Amycolatopsis sp. DEM30355 by HPLC-HRMS confirmed the presence of a lot of secondary metabolites, consistent with the anticipated variety of BGCs, together with a compound with exercise towards Gram-positive micro organism (MW of 402 Da, m/z = 403 [M + H]+, m/z = 425 [M + Na]+, (–)-tatiomicin (3)). Fermentation of Amycolatopsis sp. DEM30355, removing of the biomass, extraction of the supernatant and bioactivity guided fractionation by a number of chromatography steps resulted in a fraction which retained antimicrobial exercise and contained two carefully associated compounds. HRMS evaluation advised that these compounds had been stereoisomers of one another, the main compound exhibiting m/z = 425.1221 [M + Na]+ similar to a molecular formulation of C21H22O8 for each molecules (see ESI).
Structural willpower of the main part was initially carried out by NMR, which offered the vast majority of molecular connectivity excluding the ordering of the three contiguous quaternary centres on the C-3, C-4 and C-4a positions. Structural task was accomplished through single-crystal X-ray diffraction (XRD) evaluation, revealing a extremely oxygenated anthracenone polyketide, structurally in keeping with the BGC of curiosity, which we named (–)-tatiomicin (3) (Fig. 1)29.
NMR and HPLC experiments demonstrated that the minor compound was the C-2 epimer, able to equilibrating with (–)-(3) underneath acidic situations (see ESI).
Willpower of absolutely the stereochemistry of (–)-3 was undertaken in parallel through vibrational and digital round dichroism spectroscopies and extra single-crystal X-ray diffraction (SCXRD) experiments.
Absolute configuration willpower by vibrational round dichroism (VCD) was primarily based on a comparability of experimental and computationally predicted spectra, taking into consideration the presence of two epimers of (–)-3. Conformational evaluation (see ESI), removing of redundant geometries and closing optimization on the B3LYP/6–311 + + G(d,p) degree allowed Boltzmann-weighted VCD spectra for each epimers of (–)-3 to be constructed. The ultimate predicted spectrum was obtained by making use of a 3:1 ratio to account for the experimentally analysed combination of epimers. Numerical evaluation was used to determine settlement between experiment and concept, the neighbourhood similarity values (ΣIR = 92.0, ΣVCD = 71.2, ESI = − 57.8) suggesting an absolute stereochemical task of (2S,3S,4R,4aR,10R) (Fig. 3 and ESI)30. The task was supported by means of comparable digital round dichroism (ECD) experiments; nevertheless, on this case correlation between experiment and prediction was weaker (see ESI).
Experimental IR (prime) and VCD spectra (backside) of ( −)-tatiomicin 3 (CDCl3) with predicted spectra obtained on the B3LYP/PCM/6–311 + + G(d,p) degree of concept. VCD: Stable line = (2R,3R,4S,4aS,10S), dashed line = (2S,3S,4R,4aR,10R). Spectra have been frequency scaled Black line (σ = 0.987) to yield maximal similarity gray line between the computed and experimental VCD spectra.
An appropriate, albeit small, single-crystal of tatiomicin (3) was grown through sluggish evaporation from a benzene resolution. As a result of crystal’s dimensions, diffraction knowledge had been collected at beam line I19 on the Diamond Mild Supply utilizing synchrotron radiation at commonplace working wavelength (λ = 0.6889 Å), offering an information set of enough high quality to permit for structural affirmation. (–)-Tatiomicin (3) crystallized as an H-bonded dimer within the unit cell (Z’ = 2) together with a single molecule of solvent (benzene). To validate absolutely the stereochemical task an additional single-crystal X-ray diffraction experiment was undertaken at I19, using non-typical, longer wavelength synchrotron radiation (λ = 1.4879 Å) to boost resonant scattering contributions (additionally recognized inappropriately as anomalous dispersion). Absolutely the-structure (‘Flack’) parameter (0.05(6)) was insignificantly completely different from zero and with a small commonplace uncertainty, indicating the proper absolute configuration within the refined (2S,3S,4R,4aR,10R) construction (see ESI)29. Apparently, following intensive stereochemical debate and a number of other reported whole syntheses, absolutely the stereochemistry of the congeneric (+)-rishirilide B (2) was not too long ago revised (2S,3S,4S), matching that of (–)-(3) over the three widespread stereocentres, suggesting an analogous biosynthetic pathway for each units of pure merchandise (Fig. 4)31,32,33,34,35.
To confirm that the BGC beforehand recognized does certainly encode the biosynthetic pathway for tatiomicin (3), a excessive molecular-weight P1 synthetic chromosome (PAC) library was obtained, consisting of two,688 clones with a median insert dimension of 138 kb which contained resistant markers for kanamycin (for E. coli) and thiostreptone (for S. coelicolor). The PAC library was screened by PCR, utilizing 4 primer pairs for the putative BGC. A single PAC clone was recognized with the required PCR profile, which was then transferred into E. coli pressure ET12567/pR9604 (dam– dcm–), the plasmid was subsequently transferred into S. coelicolor M1152 through conjugation. Exconjugants containing the plasmid built-in on the chromosome had been chosen for resistance to thiostrepton. Ninety-six putatively recognized exconjugants had been arrayed into 24 properly plates and screened for the manufacturing of tatiomicin (3) by TLC, with detection primarily based on the attribute fluorescence upon UV irradiation at 365 nm. Primarily based on these screening parameters, S. coelicolor M1152::tat was recognized as a producer of tatiomicin (3) (see ESI).
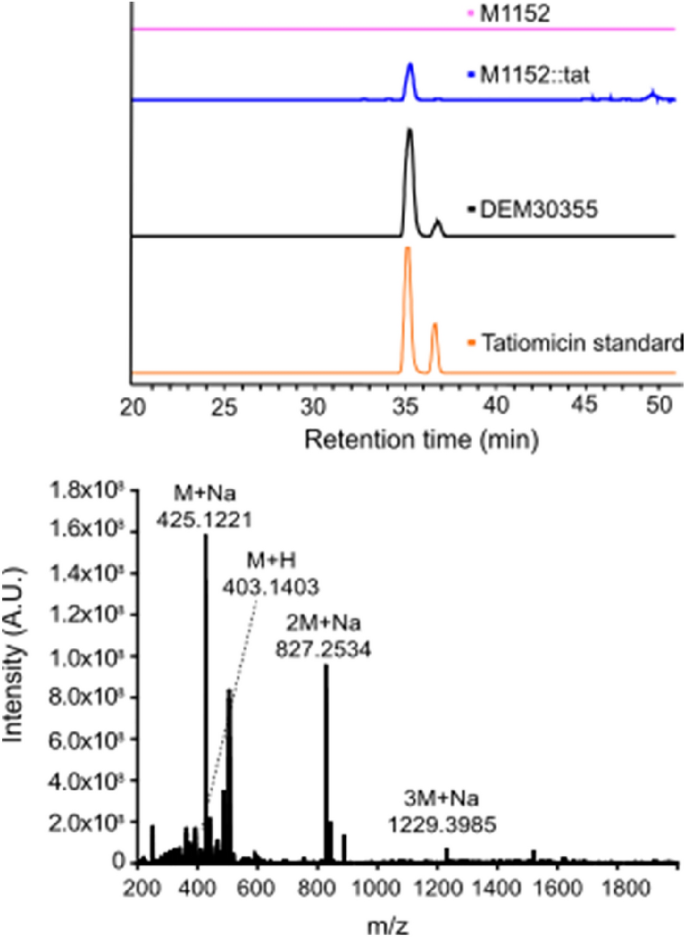
Progress of S. coelicolor M1152::tat was examined on stable medium, the agar was extracted (EtOAc) and analysed by LCMS alongside comparable fermentation extracts from each the mum or dad pressure M1152, Amycolatopsis sp. DEM30355 and a tatiomicin (3) commonplace. An LCMS peak similar to tatiomicin (3) was noticed within the extract from S. coelicolor M1152::tat however was absent in that of the mum or dad pressure M1152 (Fig. 6).
Tatiomicin (3) was subsequently remoted from the fermentation of S. coelicolor M1152::tat in liquid medium (GYMG), as demonstrated by HRMS ([M + H]+ = 403.1403), with a manufacturing degree within the heterologous host estimated at 0.57 mg/L, confirming the identification of the tatiomicin BGC (Fig. 5).
Detection of tatiomicin from the fermentation of heterologous host S. coelicolor M1152::tat. Prime) Extracted ion chromatogram (EIC) primarily based on m/z = 827.25. S. coelicolor M1152 (purple), S. coelicolor M1152::tat (blue), Amycolatopsis sp. DEM30355 (black) and tatiomicin commonplace (crimson). Backside) MS spectrum of tatiomicin (3) purified from the heterologous host S. coelicolor M1152::tat.
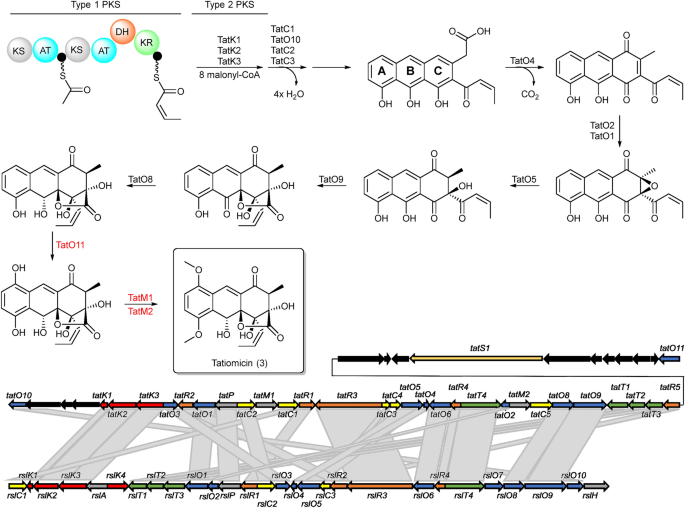
Primarily based on a comparability between the tatiomicin and rishirilide BGCs22,23,24,25,26 we suggest the next biosynthetic pathway operates for the meeting of tatiomicin (3) (See ESI). The modular sort I polyketide synthase TatS1 is probably going liable for the biosynthesis of the polyketide starter unit, cis-crotonyl-ACP, which is then elongated through the attachment of eight malonyl-CoA by minimal PKS enzymes TatK1, TatK2, and TatK3. TatC1, TatC2, TatC3 and TatO10 present shut homology to rishirilide cyclases RslC1, RslC2, and RslC3 and C9-ketoreductase RslO10, respectively. Thus, TatC1 and TatO10 seemingly act collectively to type the A hoop of tatiomicin (3), while TatC2 and TatC3 catalyse the formation of the B and C rings. Tailoring of the polyketide core seemingly entails oxidation of the C ring by TatO4, and set up of the C ring epoxide by flavin mononucleotide (FMN)-dependent monooxygenase TatO1 along with a putative flavin reductase, TatO2. Opening of the epoxide is proposed to be mediated by NADPH:acceptor oxidoreductase TatO5, adopted by the important thing Baeyer–Villiger oxidation/rearrangement managed by TatO9 and at last discount of the B ring ketone by ketoreductase TatO8.
Three extra tailoring enzymes are current within the BGC of tatiomicin (3) for which no homologues are current in that of rishirilide, TatO11, TatM1 and TatM2. TatO11 is a cytochrome p450 oxidoreductase, seemingly liable for oxidation of the A hoop to the hydroquinone type, adopted by double methylation by the 2 methyl transferases TatM1 and TatM2 to yield the finished molecule (Fig. 6).
Prime) Proposed pathway for the biosynthesis of (–)-tatiomicin (3) primarily based on homology with the biosynthetic gene cluster for the rishirilides. Enzymes proven in crimson don’t have any direct congener within the rishirilide BGC and their biosynthetic position is hypothesised, primarily based on BLAST evaluation. Backside) comparability of the rishirilide and (-) tatiomicin gene cluter primarily based on BLAST evaluation. (crimson; tatS = starter unit biosynthesis, tatK or rslK = chain biosynthesis), polyketide modification (blue; tatO or rslO = oxidoreductases, tatC or rslO = cyclases), regulation (yellow; tatR or rslR), transport (inexperienced, tatT or rslT) and others (gray; tatP or rslP = phosphorylase; tatM = methyltransferases, black; genes not assigned to the tatiomicin BGC primarily based on homology to the rishirilide BGC and proposed biosynthetic pathway)).
The enzymes TatC4 and TatC5, which aren’t current within the rishirilide cluster, encode for a dehydrogenase and a monooxygenase and are positioned within the centre of the biosynthetic gene cluster. The tatiomicin BGC incorporates all orthologous genes liable for the synthesis of rishirilide. The perform of those extra genes is due to this fact not rapid apparent and could be a results of evolutionary divergence.
(–)-Tatiomicin (3) confirmed no detectable antimicrobial exercise (MIC > 64 µg/mL) towards ten Gram-negative micro organism and two eukaryotic microorganisms (Candida spp.) (see ESI). Nevertheless, antibacterial exercise was noticed towards a sub-set of Gram-positive micro organism (MIC = 4–8 µg/mL), specifically Staphylococcus and Streptococcus species. As a result of curiosity in creating new antibiotics towards drug-resistant Staphylococcus infections, we additional evaluated (–)-3 towards a panel of MRSA scientific isolates, together with twenty-four EMRSA-15 and EMRSA-16 strains (the primary causative brokers of nosocomial epidemic MRSA bacteraemia within the UK, with resistance to penicillin, ciprofloxacin and erythromycin)36, and twelve MRSA strains remoted from Belgian, Finnish, French and German hospitals (see SI). In all instances antibiotic exercise was maintained (MIC = 4–8 µg/mL), suggesting that (–)-3 doesn’t function through a mode-of-action beforehand encountered by these strains, prompting us in direction of additional investigation.
Elucidation of the mode-of-action (MOA) for a brand new antibacterial agent is a major experimental problem. The characterization of resistance mutations might be informative, nevertheless all makes an attempt to isolate Bacillus subtilis mutants proof against (–)-tatiomicin (3) proved unsuccessful (see ESI). Additionally, no constructive responses had been seen with a panel of B. subtilis strains containing lacZ reporter genes used to point widespread antibacterial mechanisms of motion, together with: fatty acid synthesis (fabHA), DNA injury (ɸ105 prophage induction), RNA polymerase (RNAP) inhibition (helD), cell wall injury (ypuA), gyrase inhibition (gyrA), and cell envelope stress (liaI)) (see ESI)37,38,39.
As a result of presence of an, albeit electron-rich, α,β-unsaturated carbonyl moiety, we postulated that the noticed organic exercise of (–)-tatiomicin (3) might contain the covalent modification of thiol-containing enzymes by means of a conjugate or Michael addition of the thiol to the α,β-unsaturated carbonyl. Thus, (–)-tatiomicin (3) was reacted with L-cysteine hydrochloride, L-cysteine methyl ester hydrochloride and a brief thiol-containing peptide (LcrV (271–291)) as an enzyme proxy, underneath biologically related situations. In all instances thiol adducts may very well be detected by LCMS, suggesting that (–)-tatiomicin (3) might have biologically related Michael acceptor exercise (see ESI).
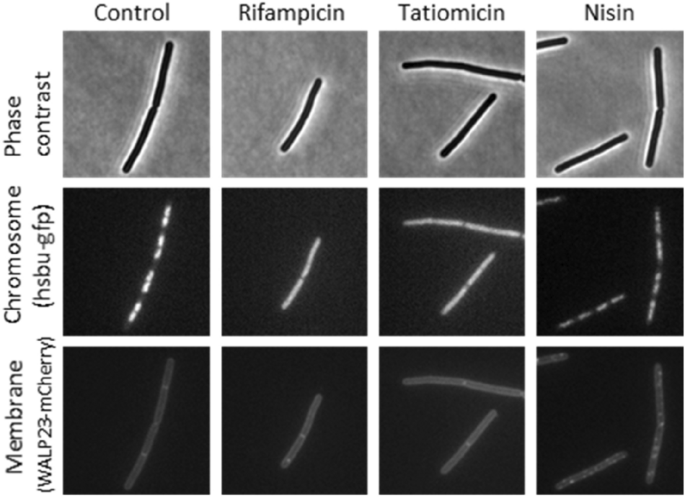
To achieve additional perception into a possible mode-of-action, we undertook a bacterial cytological profiling experiment during which antibacterial induced modifications within the morphology of check micro organism are in comparison with these induced by recognized mode-of-action antibacterials40,41. B. subtilis 168CA-CRW419 expresses two fusion proteins, HbsU-GFP and WALP23-mCherry, permitting simultaneous visualization of each the chromosomal DNA and the bacterial cell membrane by fluorescence microscopy. The cytoplasmic membrane was unaffected in contrast to within the management compound nisin, which kinds massive pores within the membrane42. Apparently, remedy with (–)-tatiomicin (3) induced chromosome decondensation in B. subtilis 168CA-CRW419, just like the results elicited by the RNAP inhibitor rifampicin (Fig. 7).
Single-cell evaluation of chromosome and membrane integrity. Section distinction (prime panels) and fluorescence microscopy of B. subtilis cells handled with numerous antibiotics (indicated above). DNA was visualized with an HsbU-GFP fusion (center panels) and the cytoplasmic membrane with a WALP23-mCherry fusion (backside panels).
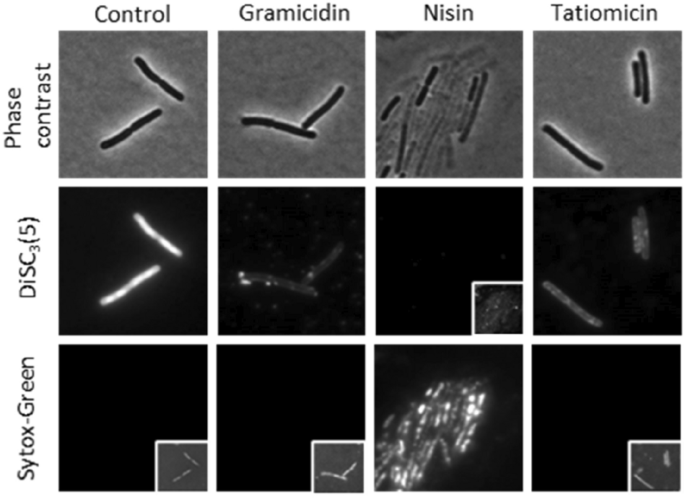
The mix of the unfavourable end result noticed with the helD reporter pressure, cell lysis after extended incubation with the compound and the lack to create resistant mutants counsel that direct RNAP inhibition is unlikely. We due to this fact tried to look at the integrity of the cytoplasmic membrane utilizing the voltage delicate dye DiSC3(5). This dye accumulates in well-energised cells within the cytoplasmic membrane15,43 however is launched upon depolarisation of the membrane, and this launch might be measured by fluorescence microscopy. DiSC3(5) is utilized in parallel with Sytox Inexperienced, a membrane-impermeable DNA stain used as a reporter for pore formation44. Upon addition of nisin, which kinds massive pores within the B. subtilis membrane42, each a lack of DiSC3(5) and uptake of Sytox Inexperienced was noticed. In distinction gramicidin, which kinds small cation-specific channels45, confirmed lack of DiSC3(5) with out Sytox Inexperienced staining. Therapy with (–)-tatiomicin (3) confirmed an analogous impact to that of gramicidin, i.e. lack of DiSC3(5) with out Sytox Inexperienced staining. Therefore tatiomicin in all probability acts to dissipate the membrane potential with out the formation of enormous pores (Fig. 8).
Single-cell measurement of membrane potential and permeability. Section distinction (prime panels) and fluorescence microscopy of B. subtilis cells stained with the voltage-sensitive dye DiSC3(5) (center panels) and the membrane permeability indicator Sytox Inexperienced (backside panels) within the presence and absence of 32 μg/mL of tatiomicin. As constructive controls, the cells had been handled with 5 μg/mL of gramicidin (membrane depolarisation with out pore formation) and 10 μM nisin (membrane depolarisation by means of pore formation). Mobile DiSC3(5) and Sytox Inexperienced fluorescence values had been quantified for cells handled with tatiomicin (32 μg/mL), gramicidin (5 μg/mL), and nisin (10 μM) (see SI).
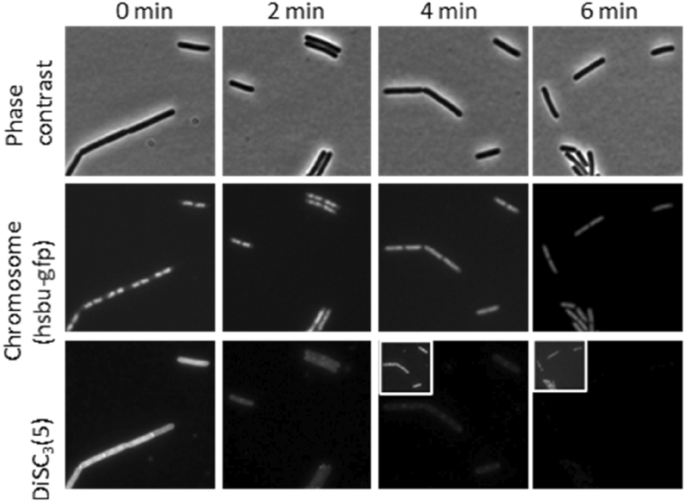
In an try to determine whether or not the noticed lack of membrane potential is a downstream impact or happens similtaneously chromosome depolarisation we carried out a time-course experiment utilizing DiSC3(5) together with a HsbU-GFP fusion to evaluate chromosome decondensation with photographs taken each two minutes. This confirmed that the lack of membrane potential occurred concurrently with the chromosome decondensation, between 2 to 4 min, suggesting that they’re carefully linked occasions (Fig. 9).
Single-cell measurement of chromosome decondensation and membrane potential in a time course experiment within the presence of tatiomicin (32 μg/mL). Section distinction (prime panels), fluorescence microscopy of B. subtilis HsbUGFP (chromosome marker) (center panel) and stained with the voltage delicate dye DiSC3(5) backside panel. Mobile DiSC3(5) fluorescence values the place quantified over time. The bar chart depicts the fluorescent depth values of particular person cells (> 30) (see SI).