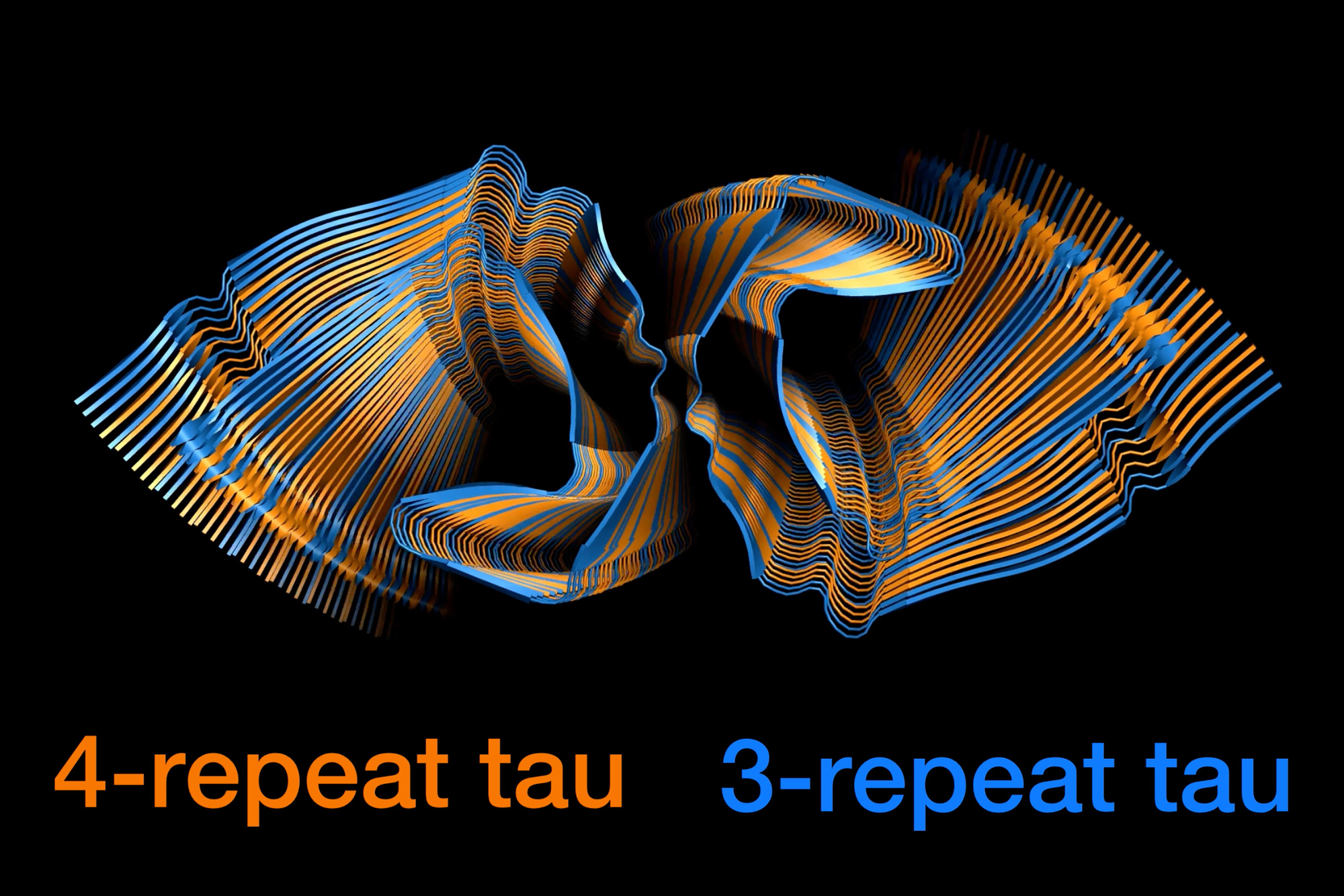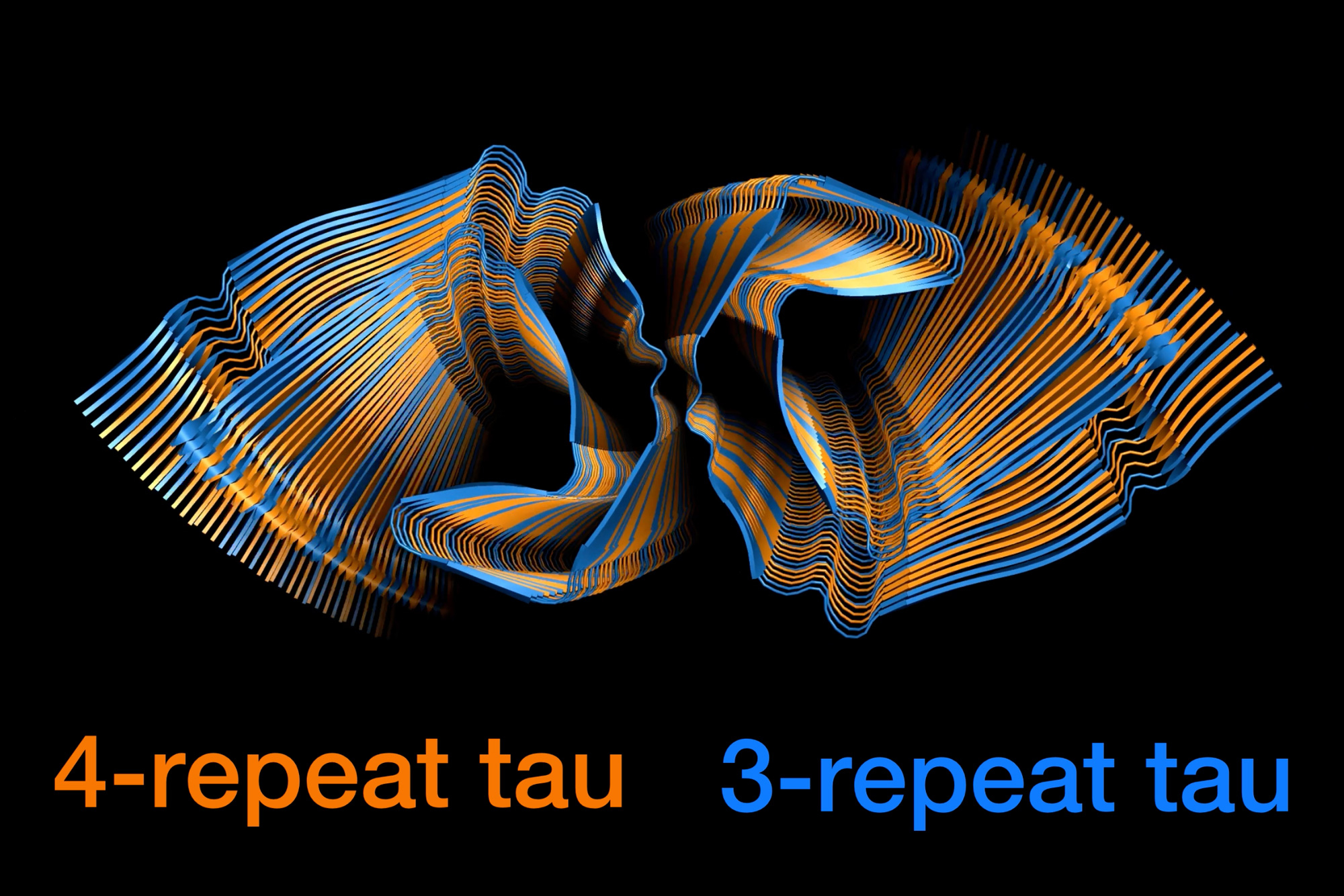
One of many hallmarks of Alzheimer’s illness is the presence of neurofibrillary tangles within the mind. These tangles, fabricated from tau proteins, impair neurons’ means to operate usually and may trigger the cells to die.
A brand new examine from MIT chemists has revealed how two varieties of tau proteins, often known as 3R and 4R tau, combine collectively to type these tangles. The researchers discovered that the tangles can recruit any tau protein within the mind, in a virtually random approach. This characteristic might contribute to the prevalence of Alzheimer’s illness, the researchers say.
“Whether or not the top of an present filament is a 3R or 4R tau protein, the filament can recruit whichever tau model is within the setting so as to add onto the rising filament. It is extremely advantageous for the Alzheimer’s illness tau construction to have that property of randomly incorporating both model of the protein,” says Mei Hong, an MIT professor of chemistry.
Hong is the senior writer of the examine, which seems as we speak in Nature Communications. MIT graduate scholar Aurelio Dregni and postdoc Pu Duan are the lead authors of the paper.
Molecular mixing
Within the wholesome mind, tau capabilities as a stabilizer of microtubules in neurons. Every tau protein is made up of both three or 4 “repeats,” every consisting of 31 amino acid residues. Irregular variations of both 3R or 4R tau proteins can contribute to a wide range of illnesses.
Continual traumatic encephalopathy, attributable to repetitive head trauma, is linked to irregular accumulation of each 3R and 4R tau proteins, just like Alzheimer’s illness. Nevertheless, most different neurodegenerative illnesses that contain tau characteristic irregular variations of both 3R or 4R proteins, however not each.
In Alzheimer’s illness, tau proteins start to type tangles in response to chemical modifications of the proteins that intrude with their regular operate. Every tangle consists of lengthy filaments of 3R and 4R tau proteins, nevertheless it wasn’t identified precisely how the proteins mix on the molecular stage to generate these lengthy filaments.
One risk that Hong and her colleagues thought-about was that the filaments may be fabricated from alternating blocks of many 3R tau proteins or many 4R tau proteins. Or, they hypothesized, particular person molecules of 3R and 4R tau would possibly alternate.
The researchers got down to discover these potentialities utilizing nuclear magnetic resonance (NMR) spectroscopy. By labeling 3R and 4R tau proteins with carbon and nitrogen isotopes that may be detected with NMR, the researchers have been capable of calculate the possibilities that every 3R tau protein is adopted by a 4R tau and that every 4R tau is adopted by a 3R tau protein in a filament.
To provide their filaments, the researchers started with irregular tau proteins taken from postmortem mind samples from Alzheimer’s sufferers. These “seeds” have been added to an answer containing equal concentrations of regular 3R and 4R tau proteins, which have been recruited by the seeds to type lengthy filaments.
To the researchers’ shock, their NMR evaluation confirmed that the meeting of those 3R and 4R tau proteins in these seeded filaments was practically random. A 4R tau was about 40 % prone to be adopted by a 3R tau, whereas a 3R tau was just a little greater than 50 % prone to be adopted by a 4R tau. General, 4R proteins made up 60 % of the Alzheimer’s illness tau filament, regardless that the pool of obtainable tau proteins was evenly divided between 3R and 4R. Inside the human mind, 3R and 4R tau proteins are additionally present in roughly equal quantities.
Such a meeting, which the researchers name “fluent molecular mixing,” might contribute to the prevalence of Alzheimer’s illness, in comparison with illnesses that contain solely 4R or 3R tau proteins, Hong says.
“Our interpretation is that this could favor the unfold and the expansion of the poisonous Alzheimer’s illness tau conformation,” she says.
Poisonous results
Working with collaborators on the College of Pennsylvania Faculty of Drugs, led by Professor Virginia Lee, the researchers confirmed that the tau filaments they generated within the lab have a construction similar to these seen in human sufferers with Alzheimer’s illness, however they don’t resemble filaments grown completely from regular tau proteins.
The tau filaments that they generated additionally replicated the poisonous results of Alzheimer’s tangles, forming aggregates within the dendrites and axons of mouse neurons grown in a lab dish.
The present paper targeted primarily on the construction of the inflexible interior core of the filaments, however the researchers now hope to additional examine the construction of the floppier protein segments that stretch out from this core. “We wish to work out simply how this protein goes from a wholesome and intrinsically disordered state to this poisonous, misfolded, and beta-sheet wealthy state in Alzheimer’s illness brains,” Hong says.
The analysis was funded by the Nationwide Institutes of Well being and the BrightFocus Basis.