Myelodysplastic syndromes (MDS) are a myeloid neoplasm characterised by clonal hematopoiesis, peripheral cytopenias, and morphologic dysplasia with a bent to progress to acute myeloid leukemia. Present therapies are tailor-made primarily based on illness danger utilizing the Revised Worldwide Prognostic System (IPSS-R) complemented by molecular knowledge and patient-related elements [1, 2]. The requirements of care for top danger MDS (HR-MDS) are hypomethylating brokers (HMA) and allogeneic hematopoietic stem cell transplantation (AHSCT), which stays the one healing possibility. HR-MDS general response charges to HMA remedy strategy 50%, though <20% obtain full response (CR) [3]. The unique AZA-001 MDS research demonstrated a median OS of 24 months, whereas a number of real-world knowledge have reported a median OS of solely 15–17 months [4, 5]. TP53 and ASXL1 somatic mutations have been related to worse OS and inferior response to HMA [6]. After HMA failure, outcomes are poor with median OS being 4–6 months and therapy choices are restricted [7,8,9]. Venetoclax is a BH3 mimetic which binds to BCL-2, an antiapoptotic protein, in the end inducing mobile apoptosis [3]. The mix of HMA and Venetoclax has turn into the usual of care treating AML sufferers unfit for intensive chemotherapy the place randomized scientific trials have demonstrated OS benefit for the mix. We in contrast outcomes of HR-MDS sufferers who’ve been handled with HMAs alone and HMA with Venetoclax, together as first line (1 L) remedy and in sequence as an add-back technique at time of HMA failure.
HR-MDS sufferers, outlined by IPSS-R by ≥ intermediate danger, handled at Moffitt Most cancers Heart who obtained an HMA as 1 L remedy after prognosis have been included on this evaluation. We in contrast outcomes of these sufferers who obtained single agent 1 L HMA, 1 L HMA/Venetoclax mixture, and HMA with Venetoclax add-back technique after HMA failure later. The choice to deal with with mixture remedy was primarily based on doctor selection. HMA failure was outlined as development on remedy or lack of response after not less than 4 cycles. Response to therapy was primarily based on the Worldwide Working Group 2006 standards. General survival was outlined from time of prognosis.
We recognized 1193 HR-MDS sufferers who obtained HMA as 1 L remedy. 1158 sufferers obtained 1 L single agent HMA (1027 obtained azacitidine and 131 obtained decitabine), and 35 sufferers obtained 1 L HMA/Venetoclax mixture (26 obtained azacitidine and 9 obtained decitabine). Among the many 1158 sufferers with 1 L HMA alone, 31 have been subsequently handled with HMA/Venetoclax mixture on the time of HMA failure with out transformation to AML. The median period of observe up from prognosis was 96 months for 1 L HMA, 15 months for 1 L HMA/Ven, and 36mo for HMA/Ven in relapsed/refractory (R/R) MDS. Desk 1A summarizes baseline scientific traits. For 1 L therapy, sufferers who obtained 1 L HMA/Venetoclax have been extra more likely to be categorized as MDS-EB2 primarily based on WHO 2016 classification and extra more likely to harbor the ASXL1 somatic mutation. The median time to provoke therapy from time of prognosis was 1 month with no distinction between the 2 arms. Venetoclax beginning dose was 400 mg PO day by day on days 1–14 of every 28-day cycle. The ven dose was adjusted as wanted for antibiotic prophylaxis per package deal insert tips. The median variety of therapy cycles administered was 5 for HMA alone and 4 cycles for HMA/Ven.
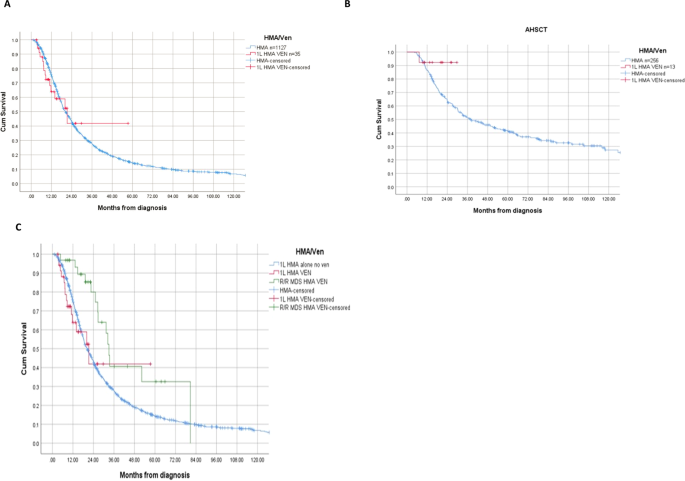
The OS was 21 months (95% CI 11–32) and 20 months (95% CI 19–22) for 1 L HMA/Venetoclax and 1 L HMA alone respectively (p = 0.86) (Fig. 1A). Among the many 269 sufferers who proceeded to allogeneic stem cell transplantation (AHSCT), 13 sufferers obtained 1 L HMA/Venetoclax. For these sufferers, the median OS was not reached in comparison with 38 months amongst those that obtained 1 L HMA alone (p = 0.20) (Fig. 1B). For sufferers who proceeded to AHSCT, the 2-year survival chance charges have been 91% and 51% for 1 L HMA/Venetoclax and HMA alone, respectively. The charges of AML transformation have been 23% and 37% for 1 L HMA/Venetoclax and HMA alone respectively (p = 0.08).
The ORR, outlined as hematological enchancment or higher, was 77% for 1 L HMA/Venetoclax in comparison with 40% for 1 L HMA alone (p < 0.005). The whole response (CR)/ marrow CR (mCR)/ partial response (PR)/ hematologic enchancment (HI) have been 34%/37%/3%/3% in comparison with 13%/11%/1%/15% for 1 L HMA/Venetoclax and 1 L HMA alone respectively (p < 0.005). Amongst sufferers with ASXL1 somatic mutation, the ORR was 87% and 32% for HMA/Venetoclax and HMA alone, respectively (p < 0.005). CR have been 44% and eight% for HMA/Venetoclax and HMA alone respectively amongst ASXL-1 mutant cohort (p < 0.005). Amongst sufferers with TP53 somatic mutation, the ORR was 75% and 44% for 1 L HMA/Venetoclax and HMA alone respectively (p = 0.038), nevertheless CR have been 25% and 17% respectively (p = 0.47) (Desk 1B).
Amongst sufferers who obtained 1 L HMA alone, 31 sufferers later obtained HMA/Venetoclax for R/R MDS. The median variety of prior 1 L HMA remedy alone was 6 cycles. For this cohort, the ORR was 61%, CR 13%, and mCR 48%. The median OS from prognosis for sufferers who obtained HMA/Venetoclax for HMA failure MDS was 33 months (95% CI 31–36). That is in comparison with 21 months (95% CI 11–32) for individuals who had 1 L HMA/Venetoclax and 20 months (95% CI 19–22) for individuals who obtained 1 L HMA alone with no subsequent Venetoclax remedy (p = 0.02) (Fig. 1C). 9 of 31 sufferers who obtained HMA/Venetoclax for R/R MDS underwent AHSCT in comparison with 22 who didn’t proceed to transplant with median OS of 31 months and 33 months, respectively (p = 0.70).
On this massive retrospective examine amongst HR-MDS sufferers, 1 L HMA/Venetoclax mixture remedy resulted in considerably increased CR charges in comparison with 1 L HMA alone. This knowledge confirms the upper response of mixture HMA/Venetoclax noticed in latest section I scientific trials and retrospective critiques. In a section 1b trial, treatment-naïve HR-MDS sufferers have been handled with Aza/Ven. Outcomes demonstrated mORR of 80%, together with CR of 40%, and mCR of 40% [10]. OS fee at 12 months was 94% for sufferers who reached a CR and 74% for individuals who reached a mCR.
An ongoing section 1b, open-label, multicenter examine carried out by Zeidan et al. evaluates the protection of Venetoclax and Azacitidine for 44 sufferers with R/R MDS. Outcomes revealed an ORR of 39% with 32% mCR and seven% CR [7]. Of these with mCR, 86% had platelet transfusion independence (TI), 71% had purple blood cell TI, and 6% had full TI. Median OS was 12.3 months for all sufferers and 14.8 months for individuals who reached mCR [7].
Forty sufferers with MDS-EB2 handled with HMA/Venetoclax on the Mayo Clinic have been included in a retrospective evaluation by Gangat et al. Sixteen sufferers have been treatment-naïve, eight have been HMA-exposed, and 16 have been HMA-refractory. All IPSS-R danger classes have been included, and 23 sufferers obtained decitabine and 17 sufferers obtained azacitadine. Thirty-eight sufferers have been included in remaining analyses, and outcomes demonstrated 30% with CR, 37.5% with mCR, and of these with mCR, 27% had HI [11]. Of the 27 sufferers with CR or mCR, 11 sufferers proceeded to AHSCT. No distinction in CR/mCR charges have been discovered between those that have been HMA-exposed, HMA-failure, or HMA naïve. HMA/Venetoclax CR/mCR was extra possible in sufferers with ASXL1, SRSF2, or IDH2 [11].
The OS profit for 1 L HMA/Venetoclax in HR-MDS sufferers can solely be addressed in context of a randomized scientific trial. We didn’t observe OS profit for the entire cohort, although restricted by small variety of sufferers handled with the mix and quick period of observe up. Our knowledge recommend promising exercise amongst those that obtained 1 L HMA/Venetoclax and proceeded to AHSCT, with a 2-year OS of 91% in comparison with a 2-year OS of 51% with 1 L HMA alone. Based mostly on our examine and knowledge from scientific trials, Venetoclax add-back technique after 1 L HMA failure has scientific exercise, together with OS profit in comparison with historic knowledge and illness management to pursue AHSCT. We assessed OS from time of prognosis, and those that obtained Venetoclax add-back technique had the longest OS. This may be attributed partially to time lead bias the place these sufferers had a response or secure illness with authentic HMA therapy alone, however these outcomes additionally pose the query of the optimum timing and sequence of utilizing Venetoclax in MDS.
Our knowledge highlights improved ORR for sufferers with the poor-prognosis related mutations of ASXL1 and TP53 handled with 1 L HMA/Venetoclax. The CR for 1 L HMA/Venetoclax vs. 1 L HMA in ASXL1 sufferers was moreover important at 44% in comparison with 8% respectively (p ≤ 0.005). Our scientific remark of upper responses with mixture remedy amongst ASXL1 mutant MDS sufferers helps the latest preclinical knowledge suggesting that ASXL1 resistance to azacitidine is mediated by overexpression of BCL-2, and addition of Venetoclax might overcome this resistance mechanism [12]. No distinction in response was noticed primarily based on different mutations, nevertheless pattern measurement of these subsets will preclude significant evaluation.
Limitations of our examine embrace its retrospective nature, small variety of sufferers handled with HMA/Venetoclax mixture, quick period of observe up for the mix arm, lack of minimal residual illness evaluation, and the doctor’s bias in administering the mix for chosen group of sufferers. No knowledge on dose adjustment and hostile occasions have been collected retrospectively for this examine goal. Lastly, we acknowledge the discrepancy between the proportion of sufferers in every group that proceeded to AHSCT.
In conclusion, the historic management arm with HMA alone is without doubt one of the largest reported and each response charges and survival are in line with earlier studies. The response charges with mixture remedy are encouraging, significantly amongst sufferers with identified low response charges to HMA alone. The two-year survival chance after AHSCT on this examine is encouraging, in addition to the exercise famous after HMA failure. Randomized scientific trials are required to adequately perceive the effectiveness of mixture remedy of HMAs and Venetoclax in addition to therapy with Venetoclax add-back technique to alter our present commonplace of care.